Aldol Reactions: Why is Excess LAH Used?
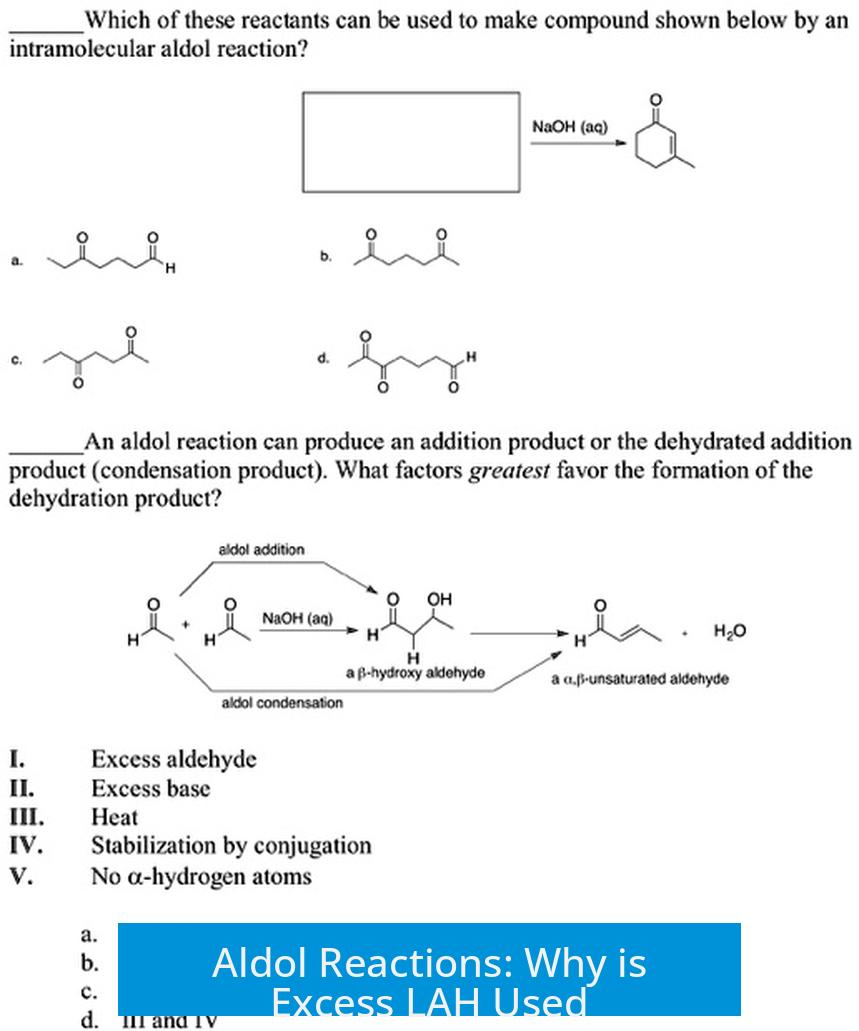
Excess lithium aluminum hydride (LAH) is used in aldol reactions primarily to compensate for hydride consumption not only in carbonyl reduction but also due to its strong basicity that leads to deprotonation of acidic groups and the formed alcohol, ensuring complete reaction.
Role of LAH as a Strong Hydride Donor and Base
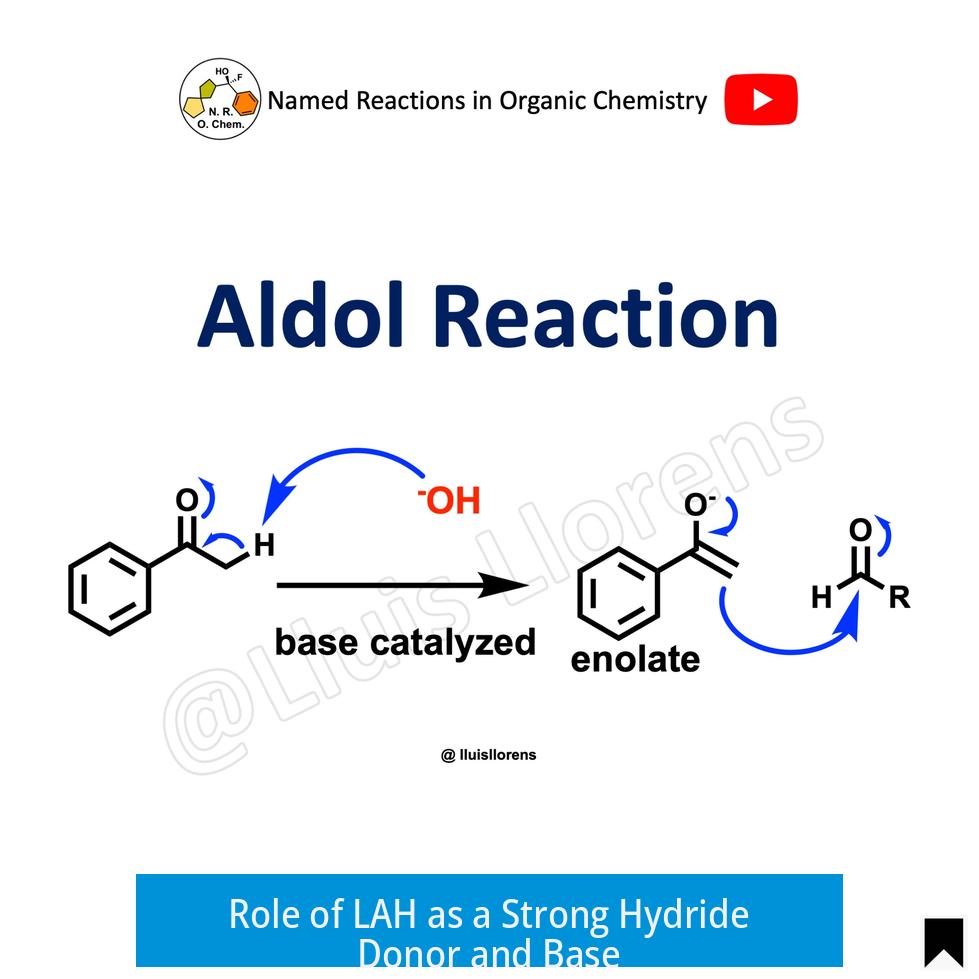
LAH provides hydride ions to reduce aldehydes and ketones formed in aldol reactions. However, it is not just a reducing agent; LAH is a potent base that deprotonates acidic groups such as hydroxyl (-OH) groups in the substrate or product.
- The deprotonation generates hydrogen gas (H2) and alkoxide ions (–O-).
- This side reaction consumes some hydride equivalents, decreasing hydride availability for the intended reduction.
Challenges in Estimating LAH Amounts

The acidity of functional groups in aldol substrates can vary widely. This variability makes stoichiometric calculation of LAH difficult. If exact equivalents are used, the reaction may remain incomplete due to hydride loss in acid-base reactions.
Thus, chemists often apply excess LAH to ensure sufficient hydride remains available to fully reduce all carbonyl species.
Hydride Consumption by the Alcohol Product
The alcohol formed after reduction also contains acidic protons. LAH can deprotonate this product alcohol further consuming hydride ions.
Practically, this means some hydride equivalents are “wasted” maintaining the alcohol as an alkoxide, which necessitates extra LAH.
Stoichiometric Considerations
| Parameter | Value/Effect |
|---|---|
| Theoretical hydride needed | ~0.25 mol LAH per mol substrate |
| Hydride loss from alcohol deprotonation | Additional ~0.25 mol LAH |
| Typical LAH used | At least 0.5 to >1 equivalent for excess |
Using more than the stoichiometric amount of LAH is common to drive the reduction to completion despite these side reactions.
Safety Precautions When Using Excess LAH
Excess LAH poses risks during workup since it violently reacts with water to produce hydrogen gas and aluminum hydroxide. This can cause explosions if quenching is done improperly.
Safe quenching involves stepwise addition of ethyl acetate, then isopropyl alcohol, followed by a mild aqueous acid to neutralize residual LAH safely.
Key Takeaways
- LAH acts both as a strong hydride donor and a strong base in aldol reductions.
- Hydride is consumed by deprotonation of acidic groups and formed alcohol.
- Exact hydride consumption is hard to predict; excess LAH ensures complete reduction.
- Typically, at least half to one full equivalent (or more) of LAH is used beyond stoichiometry.
- Careful quenching of excess LAH is critical to avoid violent reactions.
Aldol Reactions: Why Is Excess LAH Used Here?
When reducing aldol products, chemists commonly use an excess of lithium aluminium hydride (LAH), and here’s why: LAH’s nature as both a strong hydride donor and a potent base causes it to consume more reagent than the simple stoichiometry suggests, making excess indispensable for complete reactions.
If you’ve ever run an aldol reaction reduction, you might have wondered why your protocol calls for more LAH than “theoretically required.” It’s not just to be on the safe side—it’s chemistry in action. So, what’s really going on beneath the lab hood? Let’s break it down.
LAH: The Double-Edged Sword
LAH is notorious for its strong hydride donation, easily reducing carbonyl groups (like aldehydes and ketones) to alcohols. But that’s only half the story. Its basicity ranks equally high; it doesn’t just reduce—it deprotonates!
During an aldol reaction, you end up with molecules juggling various groups—carbonyls, hydroxyls, maybe even acidic sites here and there. LAH doesn’t discriminate; it grabs any acidic proton it can find. That means the hydride from LAH ends up mopping up protons from hydroxyl groups (–OH), generating hydrogen gas (H2) and alkoxides (–O−).
Think of LAH as a guest who not only eats the main course (carbonyl reductions) but also snacks on the appetizers (acidic protons). This uninvited grazing uses up precious reagent.
Why Excess LAH? The Acidity Estimation Challenge
Estimating exactly how much LAH you need is tricky—because every substrate is a little different. The acidity of groups varies with molecular context. Is that hydroxyl mildly acidic or very acidic? Is there an adjacent electron-withdrawing group boosting acidity? Each variation changes how much hydride LAH loses to deprotonations.
With so much uncertainty, chemists lean on an old adage: better too much than too little. They use an excess of LAH to cover hidden acidic “traps” in the molecule that sap hydrides away. This redundancy ensures the carbonyl groups actually get reduced, rather than leaving you half-done and disappointed.
Don’t Forget: The Product Alcohols Also Eat Hydrides
Here’s a subtle but important point. After LAH reduces your aldol carbonyl to an alcohol, that alcohol can act as a weak acid. The reactive hydride doesn’t just sit quietly—it may deprotonate the newly formed alcohol, creating alkoxides and consuming more hydride.
Imagine your reaction mix as a hydride black hole. No matter how many hydrides you toss in, some vanish by proton scavenging in unexpected places. This “hydride debt” builds up and must be compensated for.
The Math Behind Excess: Stoichiometric Considerations
| Concept | Approximate LAH Needed | Explanation |
|---|---|---|
| Theoretical minimum | 0.25 equiv | For each carbonyl group reduced, 1 hydride equivalent needed (one LAH supplies 4 hydrides) |
| Accounting for alcohol deprotonation | ≥ 0.5 equiv | Product alcohols consume hydride by deprotonation |
| Practical excess | > 1 equiv | Ensures full reduction despite acidic side reactions |
So, in practical labs, chemists often use more than one molar equivalent of LAH relative to the substrate, clearly exceeding the “ideal” stoichiometry to guarantee full conversion. Sure, this sounds wasteful, but given the cost of incomplete reactions and purifications, it’s cost-effective overall.
Safety First: Quenching Excess LAH
One cannot talk about using excess LAH without highlighting the fiery elephant in the room—safety. LAH reacts violently with water, releasing hydrogen gas and heat, sometimes explosively. You can’t just dump water in your reaction mixture. The residual hydrides in excess LAH need careful quenching.
The protocol? A slow, patient addition of ethyl acetate first, which reacts gently with leftover hydrides. Next, isopropyl alcohol is added to mop up further LAH. Only then do chemists add water or mild acid to finish quenching safely. This staged approach transforms a dangerous situation into a well-controlled one.
So yes, the choice to use excess LAH brings safety implications that must be managed with respect and rigorous technique.
The Bottom Line
LAH’s ambidextrous role as both a hydride donor and strong base means it consumes more reagent than just for reducing carbonyl groups. The unpredictable acidity of substrates and product alcohols add to this quenching experience for hydride ions. This makes using an excess vital for successful aldol reductions.
Have you faced incomplete aldol reductions despite strict stoichiometry? Did you suspect your reagent was being eaten alive? Now you know: LAH isn’t just your reducing agent but also the hydride equivalent of a hungry guest at a chemistry banquet.
Remember, using excess LAH is a practical move, not a careless extravagance. It safeguards your reaction to the finish line, but it demands respect, care, and proper quenching to avoid fiery surprises.




Leave a Comment