Distinguishing Amine, Amide, and Amino Groups
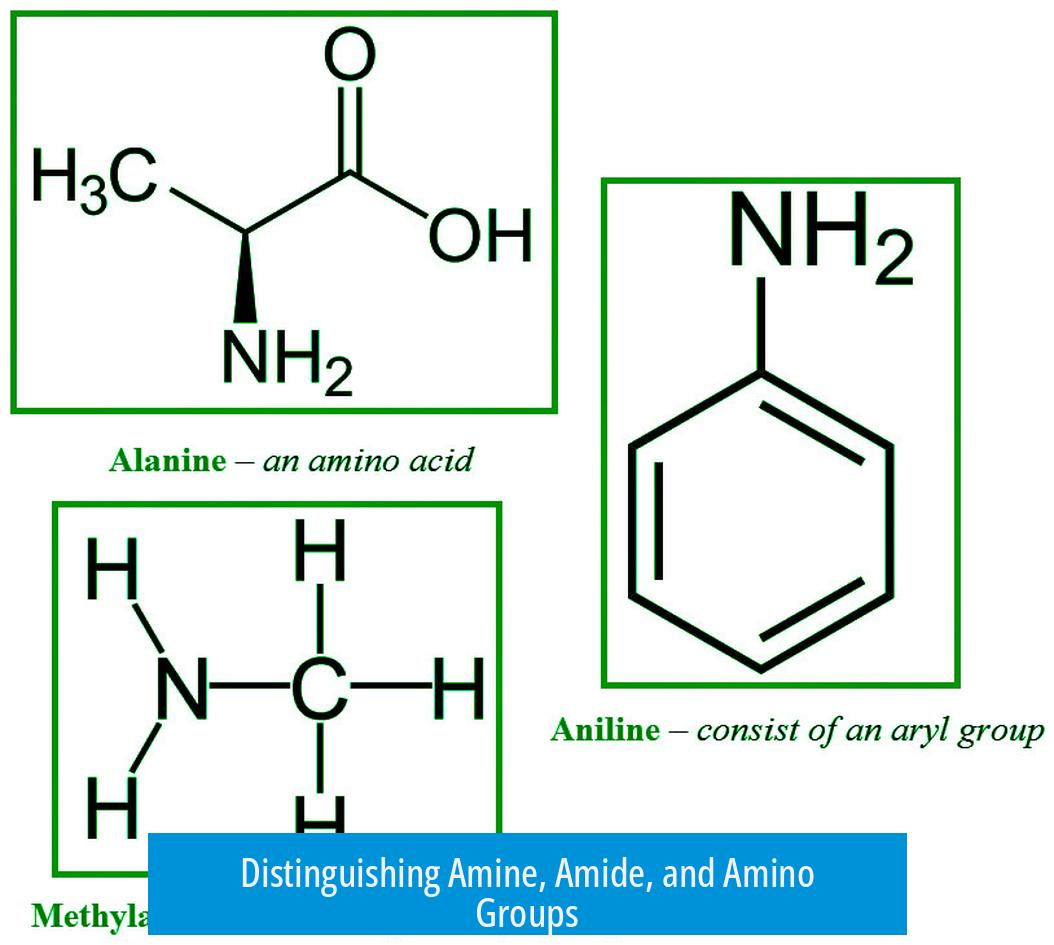
Amine, amide, and amino groups differ mainly by their oxygen content and molecular structure. Amines contain nitrogen but no oxygen. Amides have a carbonyl (C=O) group adjacent to nitrogen. Amino groups are specifically primary amines.
Core Differences
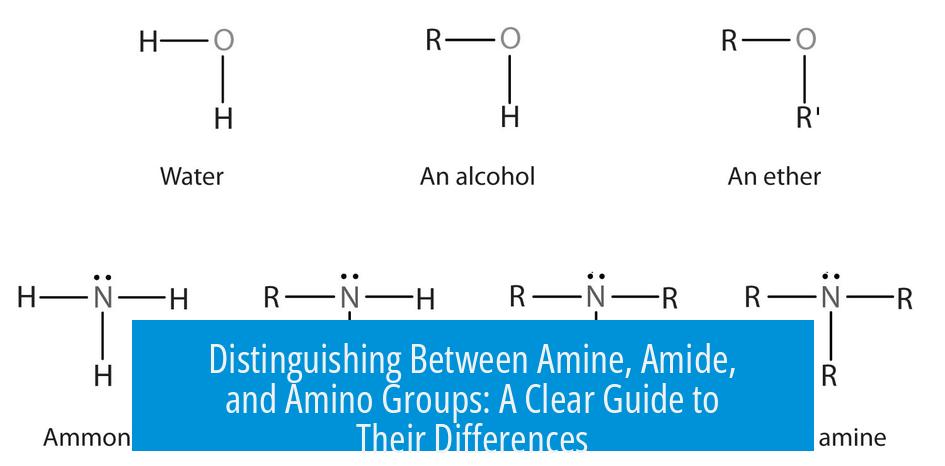
- Amine: A nitrogen-containing group without oxygen. It can be primary, secondary, or tertiary, depending on the number of alkyl groups attached to nitrogen. Example structures include R-NH2, R-NH-R, or N with three R groups (NR3).
- Amino: A primary amine subtype where nitrogen attaches to one alkyl group and two hydrogens (R-NH2). This is narrower than the general amine category.
- Amide: Consists of a carbonyl (C=O) group bonded directly to nitrogen (C=O-NH or C=O-NR). This feature introduces oxygen into the structure, differentiating it from amines.
Structural Overview
| Term | Key Feature | Example Structure |
|---|---|---|
| Amine | Nitrogen with zero formal charge, no oxygen | R-NH2, R-NH-R, NR3 |
| Amino | Primary amine only | R-NH2 |
| Amide | Carbonyl bonded to nitrogen | C=O-NH/R |
Conceptual Notes
Amines resemble ammonia (NH3) but substitute one or more hydrogens with alkyl groups (R). Amines do not contain oxygen atoms. Amides differ as they have the carbonyl oxygen bonded adjacent to nitrogen, altering reactivity and polarity.
The term “amino” specifies a primary amine. This classification matters in biochemistry, as amino groups appear frequently in amino acids where the nitrogen is bonded to one carbon and two hydrogens.
Summary of Key Points
- Amine = nitrogen group without oxygen; can be primary, secondary, or tertiary.
- Amino = specifically a primary amine (R-NH2).
- Amide contains a carbonyl (C=O) attached to nitrogen, introducing oxygen.
- Structural distinction hinges on presence of carbonyl oxygen in amides.
What is the main difference between amine and amide?
Amines do not contain oxygen, while amides have a carbonyl group (C=O) attached to the nitrogen atom.
How can I identify an amino group among amines?
Amino groups are specifically primary amines with the structure R-NH2. All amino groups are amines, but not all amines are amino.
What does ‘primary’, ‘secondary’, and ‘tertiary’ mean in amines?
- Primary amine: nitrogen bonded to one carbon (R-NH2)
- Secondary amine: nitrogen bonded to two carbons (R-NH-R)
- Tertiary amine: nitrogen bonded to three carbons (NR3)
Why is ‘amine’ sometimes written as NR2?
This represents a nitrogen atom bonded to two groups or hydrogens. It reflects the general structure of amines derived from ammonia (NH3).
How is amide structurally different from amine?
Amides have a carbonyl carbon (C=O) connected directly to the nitrogen, forming C=O-NH or C=O-NR structures. Amines lack this carbonyl group.




Leave a Comment