Understanding Reaction Mechanisms: SN1 and SN2
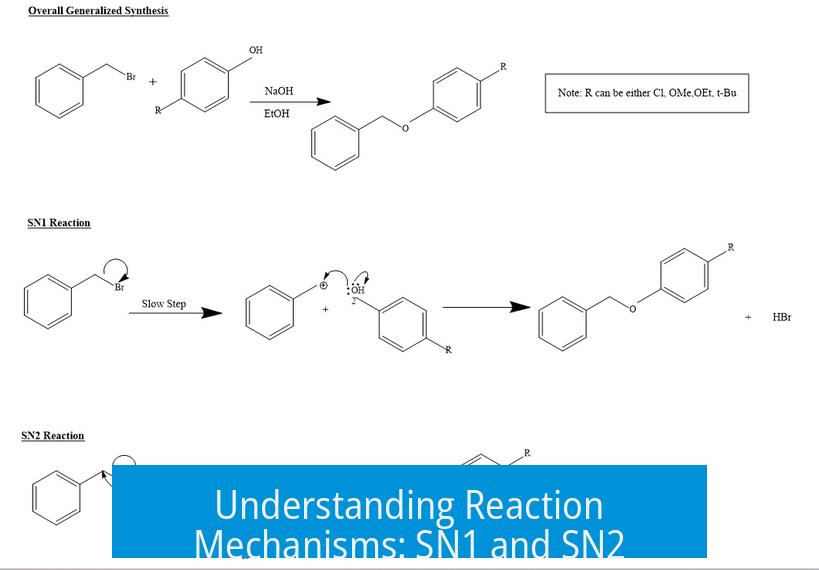
SN1 and SN2 are two fundamental nucleophilic substitution mechanisms in organic chemistry. They differ in steps, molecularity, and stereochemical outcomes.
SN1 Mechanism
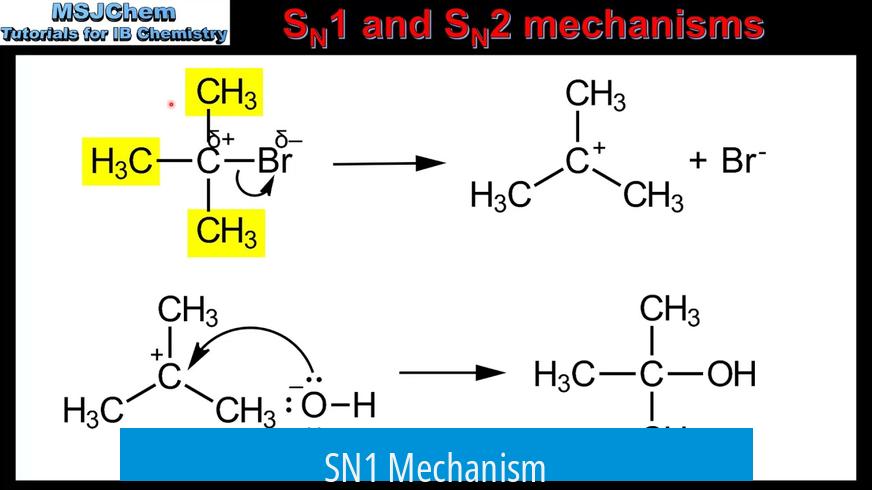
SN1 stands for unimolecular nucleophilic substitution. It occurs in two distinct steps.
- First, the leaving group departs from the substrate, forming a carbocation intermediate.
- Second, a nucleophile attacks the carbocation.
This carbocation intermediate is planar, meaning it has an equal chance of being attacked from either side. If the reactive carbon is chiral, the attack produces a racemic mixture of stereoisomers—both enantiomers form in equal amounts.
SN1 reactions favor substrates that can stabilize carbocations, such as tertiary carbons. The rate-determining step is the formation of the carbocation and depends only on the concentration of the substrate.
SN2 Mechanism
SN2 is a bimolecular nucleophilic substitution proceeding in a single concerted step.
- The nucleophile attacks the carbon atom holding the leaving group from the back side.
- Simultaneously, the leaving group departs.
This backside attack causes inversion of stereochemistry at the chiral center, known as Walden inversion.
The reaction rate depends on both substrate and nucleophile concentration, reflecting its bimolecular nature. SN2 favors primary or secondary substrates where steric hindrance is minimal.
Key Differences and Considerations
| Feature | SN1 | SN2 |
|---|---|---|
| Reaction Steps | Two-step, via carbocation intermediate | One-step, concerted |
| Molecularity | Unimolecular (rate depends on substrate) | Bimolecular (rate depends on substrate & nucleophile) |
| Stereochemistry | Racemic mix due to planar carbocation | Inversion of configuration (backside attack) |
| Preferred Substrate | Tertiary carbons (stable carbocation) | Primary or secondary carbons (less steric hindrance) |
Summary of Important Points
- SN1 proceeds in two steps, forming a planar carbocation intermediate.
- In SN1, the nucleophile attacks with no stereochemical preference, generating racemic mixtures when chiral centers are involved.
- SN2 occurs in a single step with simultaneous nucleophilic attack and leaving group departure.
- SN2 involves backside attack, causing inversion of configuration in chiral centers.
- Substrate structure influences the mechanism: tertiary favors SN1; primary favors SN2.
Can Someone Help Explain Mechanisms of Reactions and SN1 & SN2 to Me?
Ever felt like organic chemistry is a secret club where the members speak in mysterious codes like SN1 and SN2? If you’re craving a straightforward, no-nonsense explanation of these reaction mechanisms, you’re in the right place. Let’s break down mechanisms of reactions with a focus on SN1 and SN2, stripping away the jargon and replacing it with clarity—and a pinch of humor.
So, what exactly are SN1 and SN2? Here’s the nutshell: these terms describe how certain molecules swap parts during chemical reactions. SN stands for “Substitution Nucleophilic,” which means a nucleophile (a molecule eager to donate electrons) replaces a leaving group on a carbon atom. The “1” and “2” hint at the number of molecules involved in the rate-determining step and the complexity of the process.
The SN1 Story: A Two-Step Process With a Planar Twist
SN1 is a two-step, unimolecular reaction. Imagine it like a dance where the leaving group takes the first step, waving goodbye and leaving behind a carbocation—a positively charged carbon ion. This intermediate carbon is the star of the show, and it’s planar. Picture a flat stage where any dancer can approach from the front or back equally.
| Step | What Happens? |
|---|---|
| 1 | Leaving group leaves, forming carbocation intermediate |
| 2 | Nucleophile attacks the carbocation |
Since the carbocation is planar, the nucleophile can attack from either side. This geometric freedom means if the carbon is chiral (attached to four different groups), the product comes out as a racemic mixture. Simply put, you get a mix of mirror-image molecules in equal amounts.
This is a key feature that sets SN1 apart: the reaction doesn’t care which side it attacks. This lack of stereochemical preference makes SN1 reactions unique and predictable in their randomness. Isn’t chemistry fascinating?
Now, Meet SN2: The One-Step, Bimolecular Twist
SN2 is a one-step, concerted reaction. Imagine a game of tug-of-war. The nucleophile pulls the carbon just as the leaving group lets go—happening simultaneously. This means everything unfolds in one smooth, coordinated move.
During SN2, the nucleophile approaches the carbon from the back face, opposite to where the leaving group is attached. This backside attack causes the molecule to undergo an inversion of stereochemistry. Think of it as flipping an umbrella inside out.
If the carbon is chiral, SN2 will flip its configuration to the opposite stereoisomer—called inverted stereochemistry. This makes SN2 very stereospecific: it’s like the reaction has a favorite direction for its moves.
| Step | What Happens? |
|---|---|
| 1 | Simultaneous nucleophilic attack and leaving group departure |
How to Tell Them Apart? Practical Tips
- Reaction Steps: SN1 has two steps; SN2 happens all at once.
- Molecularity: SN1 depends on one molecule in the rate-limiting step; SN2 depends on two.
- Intermediate: SN1 forms a carbocation intermediate; SN2 skips intermediates.
- Stereochemistry: SN1 gives racemic mixtures; SN2 causes stereochemical inversion.
- Substrate Preference: SN1 favors tertiary carbons (bulky), while SN2 prefers primary carbons (less hindered).
For example, tertiary alkyl halides love SN1 reactions because the carbocation formed is stabilized by surrounding groups. Conversely, primary alkyl halides are SN2 stars because there’s less steric hindrance blocking the nucleophile’s attack from the back.
Why Should You Care About This?
Understanding SN1 and SN2 isn’t just academic nitpicking. These mechanisms influence how drugs behave, how plastics are made, and even how your favorite perfumes maintain their scent. Knowing which mechanism happens can help chemists design molecules that behave predictably or create compounds with specific three-dimensional shapes.
From a practical standpoint, controlling SN1 or SN2 pathways can lead to more efficient syntheses in labs and industry. For example, if a chemist wants a pure stereoisomer, avoiding SN1 (which gives racemates) and favoring SN2 reactions is crucial.
Personal Experience and Pro Tips
Many students hit a wall grasping these mechanisms. Here’s a quick tip: visualize the molecule in 3D. Grab a model kit or use an online 3D viewer. Seeing the carbocation intermediate flat in SN1 or the backside attack in SN2 can cement the concept.
Also, pay attention to reaction conditions. SN1 prefers polar protic solvents (like water or alcohol), which stabilize carbocations, while SN2 prefers polar aprotic solvents (like DMSO or acetone) to keep nucleophiles energetic and ready to attack.
Final Thoughts: SN1 vs SN2—A Tale of Two Mechanisms
SN1 is a two step, unimolecular reaction that goes through a carbocation intermediate. The first step is the loss of a leaving group, followed by nucleophilic attack of the carbocation. Since a carbocation is planar, and thus has no preferences for face of attack, you will get a racemic mix of products if the carbon is chiral.
SN2, on the other hand, is a concerted (one step), bimolecular reaction. The loss of a leaving group and attack by a nucleophile happen in the same step; this attack is a back face attack and thus results in inverted stereochemistry, if the carbon w/ the LG is chiral.
Curious to dig deeper? Check out Organic Melissa on YouTube, where detailed, lively explanations bring these reactions to life.
Still wondering “Can someone help explain mechanisms of reactions and SN1 & SN2 to me?”—consider it done! Chemistry can be clear, engaging, and even fun when you break it down step by step.
What differentiates SN1 and SN2 mechanisms in terms of reaction steps?
SN1 reactions have two steps: first, the leaving group departs, forming a carbocation. Next, the nucleophile attacks the carbocation. SN2 is a single-step reaction where nucleophilic attack and leaving group departure happen simultaneously.
How does stereochemistry change in SN1 and SN2 reactions?
SN1 forms a planar carbocation intermediate, leading to a racemic mixture if the carbon is chiral. SN2 attacks from the back, causing inversion of stereochemistry on chirality centers.
Why does SN1 produce a racemic mixture of products?
The carbocation intermediate is planar, so the nucleophile can attack from either face equally. This lack of face preference causes the formation of equal amounts of both enantiomers.
What is meant by a “back face attack” in SN2 mechanisms?
In SN2, the nucleophile attacks the opposite side of the leaving group, causing the molecule to invert its spatial arrangement. This inversion changes the stereochemistry of the chiral center.
When is an SN1 mechanism more likely than SN2?
SN1 is favored when a stable carbocation can form, often with tertiary carbons. SN2 prefers primary carbons where steric hindrance is low, as it requires a direct nucleophilic attack.




Leave a Comment