Understanding C=C and C=O Bonds from a Molecular Orbital Perspective
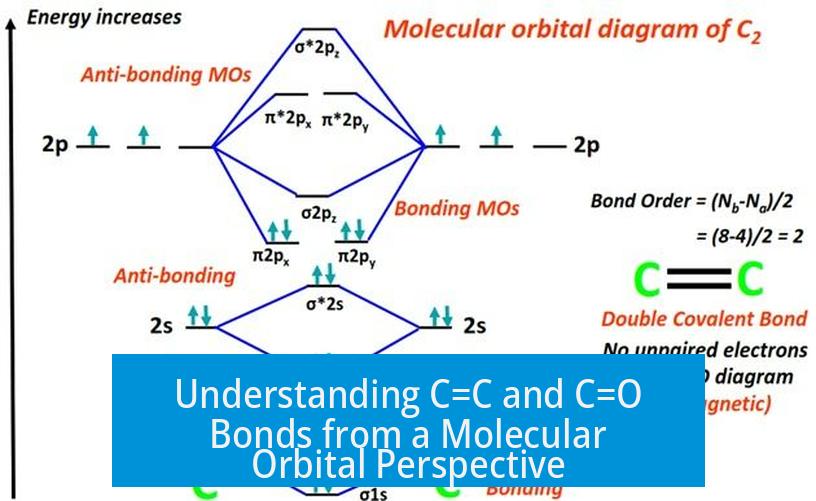
C=C and C=O bonds each involve pi bonding molecular orbitals that can exhibit both nucleophilic and electrophilic behavior depending on the chemical environment. This dual nature often leads to confusion when interpreting their reactivity. Examining these bonds through molecular orbital (MO) theory clarifies their characteristics.
Nucleophilicity of Pi Bonds
Both the carbon–carbon double bond (C=C) and the carbon–oxygen double bond (C=O) feature pi bonding orbitals containing electron pairs capable of donation.
- In the presence of a strong electrophile like HCl, the pi electrons can act as nucleophiles.
- For C=O, some may argue the nucleophilic source is the lone nonbonding pair on oxygen; however, resonance structures indicate the pi bond itself participates in electron donation.
Thus, pi bonds can supply electron density to electrophiles via their highest occupied molecular orbital (HOMO).
Electrophilicity of Pi Bonds
Conversely, both C=C and C=O can behave as electrophiles under appropriate conditions.
Their lowest unoccupied molecular orbital (LUMO), namely the pi antibonding orbital (π*), can accept electron density from nucleophiles.
Molecular Orbital Explanation
| Property | Description |
|---|---|
| HOMO (Highest Occupied MO) | Electron pair donation site, responsible for nucleophilicity |
| LUMO (Lowest Unoccupied MO) | Electron acceptance site, responsible for electrophilicity |
| C=C π* Energy | Higher than C=O π* orbital; thus, C=C is generally less electrophilic |
This means C=O’s pi antibonding orbital (π*) lies lower in energy, making it more electrophilic than C=C. Meanwhile, the presence of oxygen’s electronegativity lowers LUMO energy, enhancing electron acceptance ability.
Common Misconception
It is inaccurate to label pi bonds as solely nucleophilic or electrophilic. Their behavior depends on reactants and conditions.
They function as nucleophiles by donating electrons from their HOMO or as electrophiles by accepting electrons into their LUMO.
This duality is central to the reactivity of alkenes (C=C) and carbonyls (C=O) in organic chemistry.
Key Takeaways
- Both C=C and C=O pi bonds can act as nucleophiles and electrophiles.
- Nucleophilicity arises from electron donation via the HOMO.
- Electrophilicity comes from electron acceptance into the LUMO (π* orbital).
- C=O is generally more electrophilic due to lower π* orbital energy.
- Pi bonds should not be simplistically classified as only nucleophilic or electrophilic.
Clearing the Fog: Understanding C=C and C=O Bonds in Molecular Orbital Terms

Are C=C and C=O bonds nucleophilic or electrophilic? The short answer is—they can be both, depending on the context and molecular orbital interactions. If you jumble these ideas without a molecular orbital lens, things get tangled fast. But peel back the layers through orbitals, and the confusion fades.
Let’s walk through this common puzzle in chemistry, focusing on the molecular orbital perspective to clarify why these key double bonds defy simple labels.
Pi Bonds: Friends with Multiple Facets
At first blush, pi bonds in both C=C (carbon-carbon double bond) and C=O (carbon-oxygen double bond) look like classic nucleophiles. They hold a pi bonding molecular orbital (MO) filled with electrons that can be donated. Think of them as eager sharers—ready to hand over electrons to something electron-hungry.
- For instance, when faced with a strong electrophile like HCl, both C=C and C=O can donate their pi electrons.
- Particularly in C=O, some might argue that the lone pair on oxygen steals the nucleophile spotlight. Yet the resonance structures highlight the pi bond’s direct involvement in reactivity.
So these pi bonds actively take roles as nucleophiles in many reactions. But wait—there’s a twist.
Flip the Coin: Electrophilicity Also Applies
Many learners trip over this: If pi bonds are nucleophilic, how can they also be electrophilic? The magical answer lies in the molecular orbital world.
Both C=C and C=O bonds can act as electron acceptors under the right circumstances.
Imagine an electrophile coming into play but from the perspective of orbital energies. These pi bonds have unoccupied antibonding orbitals, termed pi-star (π*), which can accept electron density. This acceptance turns the pi bond into an electrophile momentarily.
The Molecular Orbital Playbook: HOMO and LUMO in Action
Here’s where everything clicks:
- HOMO (Highest Occupied Molecular Orbital): This is the orbital where the molecule’s most energetic electrons live. When a molecule acts as a nucleophile, it donates electrons from its HOMO.
- LUMO (Lowest Unoccupied Molecular Orbital): This orbital is ready to accept electrons. When a species acts as an electrophile, it accepts electrons into its LUMO.
In both C=C and C=O molecules, the HOMO often represents the pi bonding orbital, and the LUMO is the pi-star orbital.
One telling difference: The pi* orbital’s energy level in C=C is higher than in C=O. This matters because a higher-energy LUMO is less inclined to accept electrons, making the C=C less electrophilic. Meanwhile, the lower-energy pi* in C=O means it more readily grabs electrons, enhancing its electrophilic character.
Does that mean C=O is always electrophilic? Nope. It depends on the reaction environment and the interacting partner.
Don’t Box Pi Bonds as Only One or the Other
Here’s a crucial insight to untangle misconceptions:
Labeling pi bonds strictly as nucleophilic or electrophilic is a trap.
These roles aren’t fixed badges but flexible hats worn depending on molecular orbitals and reaction partners.
It’s a common mistake to say, “C=C is nucleophilic, and C=O is electrophilic,” as if these bonds live in isolated worlds. Reality? They operate on a spectrum, shifting sides with subtle changes in conditions.
Benefits of This Molecular Orbital Perspective
Understanding this dual nature prevents oversimplifications that can lead to wrong predictions in organic mechanisms.
Take nitrile addition to carbonyls, or electrophilic attack in alkenes. Knowing which orbital plays the starring role helps design better, more efficient syntheses and rationalize outcomes.
It even aids with computational chemistry models, where HOMO-LUMO gaps inform reactivity and stability predictions.
Practical Example: The HCl Reaction
Let’s imagine HCl approaching a molecule with either a C=C or C=O bond.
- The molecule donates its pi electrons from the HOMO to the electrophilic hydrogen (nucleophilic behavior).
- Simultaneously, the pi* orbital of the molecule might accept electron density if the Cl- ion attacks afterward, displaying electrophilic tendencies.
This sequence makes perfect sense only if you embrace the molecular orbital interactions rather than oversimplified bond behaviors.
Stories from the Lab: Why Students Struggle
Many students face confusion because textbook diagrams often separate nucleophilicity and electrophilicity into neat categories. But molecules don’t carry sticky notes identifying their roles.
Think of these pi bonds as skilled actors switching between roles dynamically rather than one-trick ponies. This mindset opens new doors for understanding organic chemistry deeply.
Recommendations for Learners and Educators
- Focus on molecular orbital theory when studying bonding and reactions.
- Remember that HOMO and LUMO concepts explain dual reactivity.
- Avoid oversimplified classifications of bonds.
- Use computational tools or orbital diagrams as visual aids.
- Practice with reaction examples involving electron flow from both nucleophile and electrophile viewpoints.
By adopting this view, the “confusion” about C=C and C=O double bonds in terms of molecular orbitals becomes a fascinating tale of electron dance, not a chemistry nightmare.
In Closing
Next time you encounter a reaction involving C=C or C=O bonds, ask yourself: “Is this molecule donating electrons from its HOMO or accepting electrons into its LUMO right now?” That question unlocks the mystery behind why these pi bonds wear many hats.
Ultimately, clear thinking about molecular orbitals dismantles traditional misconceptions and empowers chemists to predict and manipulate chemical behavior with confidence.
What determines if a C=C or C=O bond acts as a nucleophile?
Both C=C and C=O can donate electrons from their pi bonding molecular orbitals. The highest occupied molecular orbital (HOMO) provides these electrons when reacting with strong electrophiles.
How can C=C and C=O bonds be electrophilic?
Both bonds have a lowest unoccupied molecular orbital (LUMO) known as the pi* orbital. They can accept electrons into this orbital under the right conditions, showing electrophilic behavior.
Why is it wrong to label pi bonds as only nucleophilic or electrophilic?
Pi bonds can act as both nucleophiles and electrophiles depending on the situation. It’s incorrect to assign them exclusively to one role since their HOMO and LUMO energies allow dual behavior.
How does the energy of the pi* orbital affect electrophilicity of C=C versus C=O?
The pi* orbital of C=C is typically higher in energy. This makes C=C less electrophilic compared to C=O, whose lower-energy pi* better accepts electron pairs.




Leave a Comment