Difference Between Alkanes and Alkyls, Alkenes and Alkenyls, and Alkynes and Alkynyls

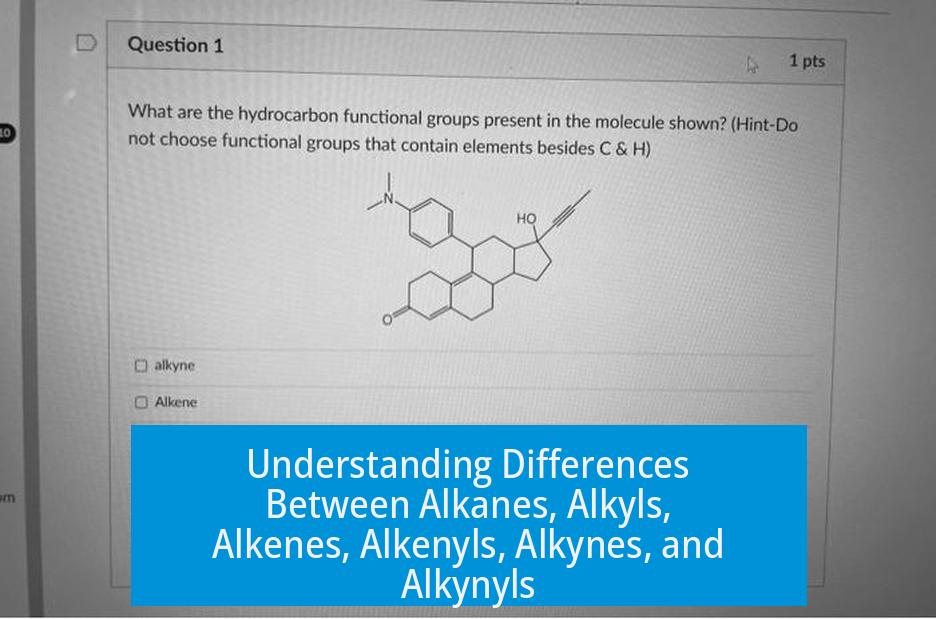
Alkanes, alkenes, and alkynes are types of hydrocarbons characterized by their carbon-carbon bonding (single, double, and triple bonds respectively), while alkyls, alkenyls, and alkynyls refer to their corresponding substituent groups attached to a molecule.
Definitions and Nomenclature
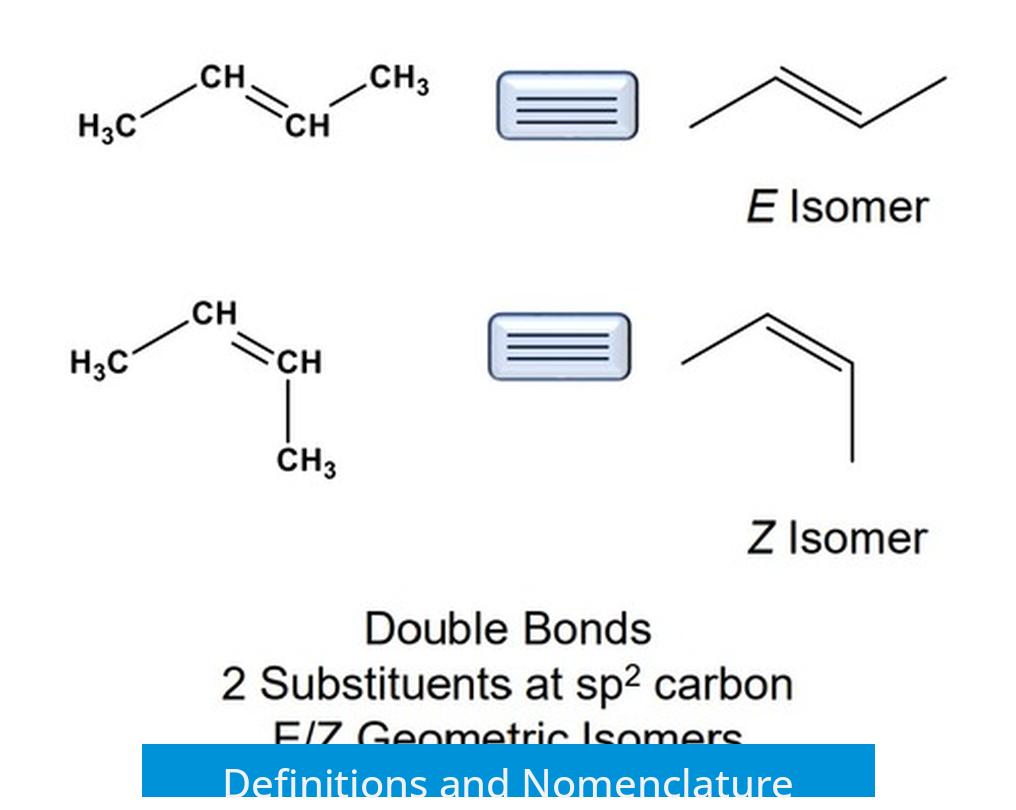
Alkanes, alkenes, and alkynes are hydrocarbons defined by the type of bond between carbon atoms:
- Alkanes have carbon-carbon single bonds.
- Alkenes contain at least one carbon-carbon double bond.
- Alkynes feature at least one carbon-carbon triple bond.
In contrast, alkyls, alkenyls, and alkynyls are substituent groups derived by removing one hydrogen atom from an alkane, alkene, or alkyne molecule respectively. They function as “side chains” attached to a parent molecule.
Nature of Substituent Groups
The suffix -yl signifies that the species is a group attached to a larger structure. For example:
- Methyl (CH3−) is an alkyl group derived from methane (an alkane).
- Vinyl (CH2=CH−) is an alkenyl group from ethene (an alkene).
- Ethynyl (CH≡C−) is an alkynyl group from ethyne (an alkyne).
This distinction can be loosely compared to nouns and adjectives: alkanes, alkenes, and alkynes resemble nouns (the main molecules), while alkyls, alkenyls, and alkynyls work like adjectives, describing a part attached to a larger molecule.
Chemical Structure and Bonding
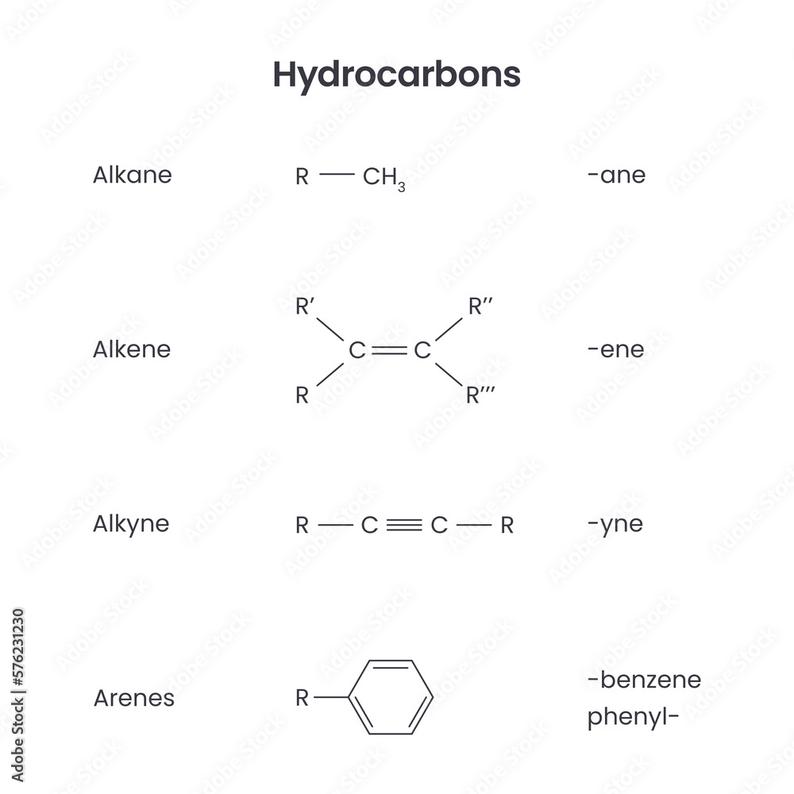
| Type | Bond Type | Example | Substituent Group |
|---|---|---|---|
| Alkane | Single (C−C) | Methane (CH4) | Methyl (CH3−) |
| Alkene | Double (C=C) | Ethene (CH2=CH2) | Vinyl (CH2=CH−) |
| Alkyne | Triple (C≡C) | Ethyne (CH≡CH) | Ethynyl (CH≡C−) |
The key difference between the parent compounds lies in their carbon-carbon bond type. Alkyl, alkenyl, and alkynyl groups carry the corresponding bonding motif but are attached as substituents.
Implications of Bonding Differences
The bonding type affects hydrogen content and reactivity significantly. Alkanes are saturated hydrocarbons with the maximum number of hydrogens. Alkenes and alkynes are unsaturated, having fewer hydrogens due to double or triple bonds.
These differences influence chemical behavior:
- Alkanes show relatively low reactivity, mainly undergoing substitution or combustion.
- Alkenes are more reactive, readily undergoing addition reactions at double bonds.
- Alkynes possess high reactivity due to the triple bond, and can undergo multiple addition and substitution reactions.
Substituent groups retain characteristics dictated by their bonding, affecting the way they interact chemically once attached to larger molecules.
Example: Methyl Chloride
Methyl chloride (CH3Cl) is a compound derived from methane, an alkane. Here, the methyl group acts as an alkyl substituent attached to chlorine, illustrating how alkyl groups form parts of compounds by substitution.
Key Takeaways
- Alkanes, alkenes, and alkynes are hydrocarbons with single, double, and triple carbon-carbon bonds.
- Alkyl, alkenyl, and alkynyl groups are substituents derived by removing one hydrogen from their parent hydrocarbons.
- The suffix -yl indicates a group attached to a larger molecule.
- Bond type defines hydrogen content and chemical reactivity differences.
- Substituent groups influence the properties of the molecules they attach to.
Difference Between Alkanes and Alkyls, Alkenes and Alkenyls, and Alkynes and Alkynyls
What’s the real difference between alkanes and alkyls, alkenes and alkenyls, and alkynes and alkynyls? Simply put: alkanes, alkenes, and alkynes are full-fledged hydrocarbons featuring single, double, and triple bonds respectively, while alkyls, alkenyls, and alkynyls are their corresponding substituent groups attached to other molecules.
Sounds straightforward? Hang tight because the chemistry behind the scenes adds some juicy layers.
The trio of alkanes, alkenes, and alkynes are organic hydrocarbons. That’s a fancy way of saying they only contain carbon and hydrogen atoms. Alkanes boast single bonds, making them the “chill folks” of hydrocarbons — saturated and steady. Alkenes come with double bonds, turning up the reactivity a notch. Alkynes are the daredevils with triple bonds, sporting even higher reactivity.
In contrast, the terms alkyls, alkenyls, and alkynyls refer to substituent groups derived from these hydrocarbons. Imagine ripping off a hydrogen from an alkane, and voilà, you get an alkyl group. Same goes for alkenes and alkynes producing alkenyl and alkynyl groups by losing a hydrogen atom.
Why the “-yl” suffix?
Here’s a neat linguistic trick. The suffix “-yl” acts like a tiny flag telling you “I’m attached to something else.” So “methyl” is an alkyl derived from methane (an alkane) but now hanging out as a substituent. For example, methyl chloride (CH3-Cl) is an alkyl chloride, where a methyl group attaches to chlorine.
Think of it this way:
- Alkane = full hydrocarbon noun (methane, ethane, propane, etc.)
- Alkyl = the adjective/substituent form (methyl, ethyl, propyl, etc.)
Same thing applies to alkenes/alkenyls and alkynes/alkynyls. It’s almost like grammar lesson meets chemistry lab.
How do these bonding differences affect chemistry?
The core difference between these families is the bond type between carbon atoms:
| Hydrocarbon Type | Bond Between Carbons | Hydrogen Count | Reactivity Level |
|---|---|---|---|
| Alkane | Single bond (C-C) | Maximum hydrogen saturation | Least reactive (stable) |
| Alkene | One or more double bonds (C=C) | Fewer hydrogens than alkanes | Moderate reactivity |
| Alkyne | One or more triple bonds (C≡C) | Even fewer hydrogens | Most reactive among the three |
Notice something? The number of hydrogens drops as the bond order climbs from single to triple. But don’t mistake hydrogen count as the defining feature — it’s really about how carbons share their electrons. This difference affects geometry, electron distribution, and ultimately, how these molecules act chemically.
Reacting like champs
Because alkenes and alkynes have unsaturated bonds (double or triple), they generally show more reactivity.
- Alkanes can be seen as the family’s couch potatoes—stable and pretty unreactive.
- Alkenes have more ‘edge’ and snap up reactions like additions due to their double bonds.
- Alkynes, with their triple bonds, are even more eager to get involved in chemical adventures.
This reactivity pattern also reflects acidities: alkynes are more acidic than alkenes, which are more acidic than alkanes. The higher s-character of the carbon’s hybrid orbitals in alkynes stabilizes the conjugate base, nudging acidity up.
Real-Life Examples and Applications
Let’s get practical. Alkanes, those saturated hydrocarbons, serve as primary fuels and lubricants. They’re what powers your car or warms up your home.
Alkenes come in handy in the production of plastics like polyethylene. Their double bonds allow them to link up into long chains under the right conditions. Ever packaged a sandwich in cling wrap? Thank alkenes.
Alkynes, while less common, are useful in synthesizing complex organic molecules, including pharmaceuticals. Their triple bonds add a versatility chemists love.
Substituent groups like alkyls show up everywhere in organic chemistry as building blocks or modifiers. For example, adding a methyl group (an alkyl) to a drug molecule can change how the body absorbs it or how it tastes. Easy to get excited about a tiny CH3!
Why knowing the difference matters?
Imagine you’re cooking and the recipe calls for a teaspoon of salt (alkane). Instead, you toss in a pinch of chili powder (alkyl). Your dish now has a totally different flavor. Similarly, in chemistry, confusing alkanes with alkyl groups or alkenes with alkenyl substituents can lead to misunderstandings in structure, reactivity, or synthesis routes.
Understanding these terms helps chemists accurately describe molecules and predict how they will behave. It’s the language that prevents mix-ups in labs and textbooks worldwide.
Summary: Breaking it down simply
Alkanes, alkenes, and alkynes are entire molecules made of carbon and hydrogen with single, double, and triple bonds, respectively. They’re nouns, if you will. When you remove one hydrogen and want to describe that leftover piece as a group attached to another molecule, you switch to alkyl, alkenyl, or alkynyl forms, the adjectives of the hydrocarbon world.
The “-yl” suffix is a handy cue signaling a substituent. Bond types matter because they influence numbers of hydrogens, molecule shape, reactivity, and even acidity.
Next time you see a word like “methyl,” “vinyl” (an alkenyl), or “ethynyl” (an alkynyl), remember: these are small groups derived from their parent hydrocarbons but acting as guests on bigger molecular parties.
So what’s your favorite—steady alkanes, edgy alkenes or thrill-seeking alkynes? Chemistry’s got a whole family for every personality.
What is the main difference between alkanes and alkyls?
Alkanes are hydrocarbons with single bonds between carbons. Alkyls are groups derived from alkanes by removing one hydrogen. Alkyls act as substituents attached to other atoms or groups.
How do alkenes differ from alkenyl groups?
Alkenes have carbon-carbon double bonds and exist as independent molecules. Alkenyl groups are parts of molecules, derived from alkenes, and attached to other atoms or groups.
Why do alkynes and alkynyls differ structurally?
Alkynes contain a carbon-carbon triple bond. Alkynyls are substituent groups from alkynes formed by removing a hydrogen atom, allowing attachment to other structures.
What does the “-yl” suffix signify in alkyl, alkenyl, and alkynyl?
The “-yl” suffix indicates a group that is attached to another atom or molecule. It shows that these are substituent groups derived from their parent hydrocarbons.
Does the number of hydrogen atoms define the difference between these groups?
Hydrogen count varies with bonding but does not define the group. The key difference lies in the type of carbon-carbon bond—single, double, or triple—and whether the group is attached as a substituent.




Leave a Comment