Difference Between SN2 and SN1 Reactions: A Beginner’s Guide
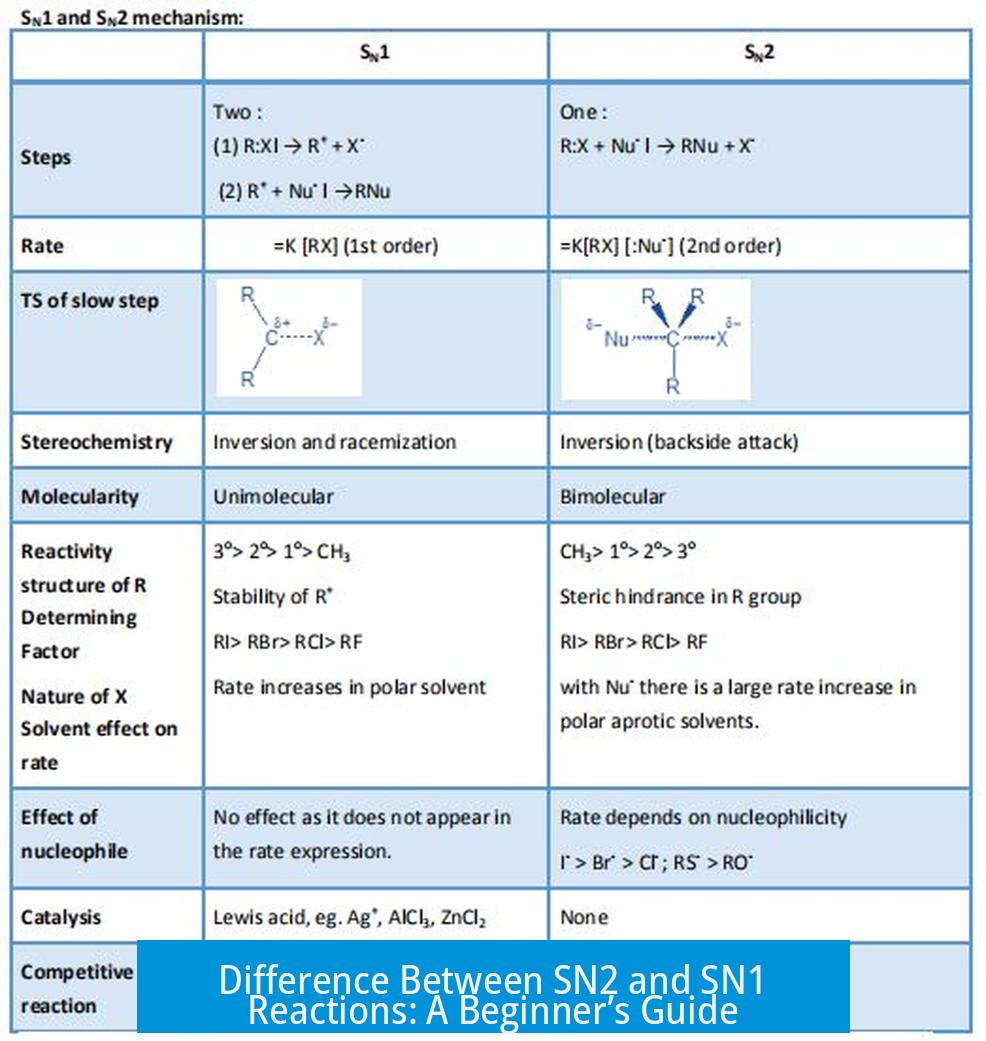
SN1 and SN2 reactions are two main types of nucleophilic substitution reactions differing in their mechanisms, steps, intermediates, and stereochemical outcomes. Both replace a leaving group in a molecule with a nucleophile, but they do so through distinct processes. Understanding these differences helps interpret many organic reactions.
What Are Substitution Reactions?
Substitution reactions involve swapping a less reactive group, called the leaving group, with a more reactive nucleophile. These reactions are key in modifying organic compounds, useful in pharmaceuticals and chemical synthesis.
Overview of Nucleophilic Substitution

Nucleophilic substitution replaces a leaving group with a nucleophile—an electron-rich species seeking positive centers.
- SN1: “Substitution Nucleophilic Unimolecular” involves one molecule in the slow step.
- SN2: “Substitution Nucleophilic Bimolecular” involves two molecules simultaneously in the rate-determining step.
SN1 Reaction Explained
Mechanism and Steps
- Bond Cleavage: The leaving group detaches first, forming a carbocation intermediate. This step controls the rate.
- Carbocation Intermediate: The positively charged carbon is unstable but stabilized by molecular structure. More substituted carbocations are more stable, reacting faster.
- Nucleophile Attack: The nucleophile quickly bonds to the carbocation, completing substitution.
Applicable Substrates
- Favored on tertiary alkyl halides and carbocations stabilized by resonance.
- Secondary and tertiary alcohols typically undergo SN1 with hydrogen halides.
Stereochemical Outcome
When the reaction involves chiral carbons, SN1 creates a racemic mixture due to the planar carbocation intermediate, allowing nucleophiles to attack from either side.
Factors Affecting SN1 Rate
- Slower with strong bases; faster with weak bases or neutral nucleophiles.
- Favored in polar protic solvents like water or alcohols that stabilize intermediates.
SN2 Reaction Explained
Mechanism and Steps
- Nucleophile Approach: The nucleophile attacks the molecule directly opposite the leaving group, creating a transition state where both are partially attached.
- Simultaneous Bond Formation and Breakage: The nucleophile bonds while the leaving group detaches.
- Product Formation: The nucleophile completes bonding, displacing the leaving group.
Applicable Substrates
- Most common with methyl, primary, and some secondary alkyl halides.
- Tertiary alkyl halides rarely undergo SN2 due to steric hindrance.
- Primary alcohols generally react via SN2 mechanisms with hydrogen halides.
Stereochemical Outcome
SN2 reactions invert the stereochemistry at the reactive carbon, often called “Walden inversion.”
Reaction Features
- One-step mechanism involving a bimolecular rate-determining step.
- Rate depends on nucleophile concentration and substrate.
- Strong nucleophiles and good leaving groups increase reaction rate.
Table of Key Differences
| Feature | SN1 | SN2 |
|---|---|---|
| Rate Determining Step | Unimolecular (only substrate involved) | Bimolecular (substrate and nucleophile involved) |
| Mechanism Steps | Two-step with carbocation intermediate | One-step concerted process |
| Intermediate | Carbocation formed | No carbocation intermediate |
| Stereochemistry | Racemization (loss of stereochemical information) | Inversion of configuration |
| Substrate Preference | Tertiary & stabilized carbocations | Methyl, primary, and secondary with low hindrance |
| Effect of Nucleophile | Weak nucleophiles tolerated | Strong nucleophiles needed |
Nucleophile vs. Electrophile in Substitution
A nucleophile has extra electrons; it donates them to form bonds. It is often negatively charged or neutral but has lone pairs.
An electrophile lacks electrons and seeks them. It usually carries a positive charge or partial positive charge.
In nucleophilic substitution, the nucleophile attacks the electrophilic carbon atom bonded to the leaving group.
Key Points to Remember
- SN1: Two steps, carbocation intermediate, racemization, favored by tertiary substrates, weak nucleophiles, and polar protic solvents.
- SN2: One step, backside attack, inversion of stereochemistry, favored by methyl/primary substrates, strong nucleophiles, and polar aprotic solvents.
- Choice of mechanism depends on substrate structure, nucleophile strength, solvent, and reaction conditions.
- Both types replace a leaving group but do so via very different paths.
Difference Between SN2 and SN1 Reactions / Nucleophilic Substitution for a Complete Beginner
If you’ve ever wondered how chemists swap parts of molecules to make something new, you’ve bumped into the world of nucleophilic substitution. Here, we’ll cut through the jargon and unravel the difference between SN2 and SN1 reactions, the two main pathways molecules take when swapping groups. Ready? Let’s dive!
What’s Nucleophilic Substitution Anyway?
Nucleophilic substitution is a fancy way of saying one group in a molecule gets replaced by another group called a nucleophile. Imagine a group leaves a party, and a new guest—the nucleophile—takes their spot. This swap is central in organic chemistry, helping craft everything from pharmaceuticals to plastics.
Two dominant types rule this swapping game: SN1 and SN2. Both aim for the same goal but march to different beats.
The Big Picture: SN1 vs. SN2
| SN1 | SN2 |
|---|---|
| Two-step reaction | One-step reaction |
| Involves a carbocation intermediate | No carbocation formed |
| Unimolecular rate (depends on one molecule) | Bimolecular rate (depends on two molecules) |
| Racemic mixture formed if chiral center involved | Inversion of configuration (stereochemistry flips) |
| Favored by tertiary carbons | Favored by primary or methyl carbons |
SN2 Reaction: The Swift One-Step Dance
SN2, or Substitution Nucleophilic Bimolecular, is like a well-directed dance where two partners—the nucleophile and the molecule containing the leaving group—come together in a single, smooth move. The nucleophile attacks the backside of the molecule (opposite the leaving group), pushing the leaving group out in one swift step.
This mechanism usually happens with less bulky molecules such as methyl, primary, and sometimes secondary alkyl halides. Tertiary ones are too crowded to let the nucleophile slip in easily.
Since the nucleophile attacks from the opposite side, the molecule’s chiral center flips like a pancake. If your molecule is chiral, expect an inversion of stereochemistry, which scientists call a Walden inversion. So, the final product looks like a mirror image of the starting molecule.
Want to picture it? Think of your nucleophile as a sneaky burglar knocking on the back door to kick out the tenant (leaving group) in a single coordinated move.
What Drives SN2?
- Strong nucleophiles are key players.
- Less steric hindrance—that is, molecules with less crowding near the site—makes it quicker.
- Good leaving groups speed things up.
- Polar aprotic solvents—like acetone—help the nucleophile stay eager.
SN1 Reaction: The Two-Step Strategy with a Guest Star
SN1 is shorthand for Substitution Nucleophilic Unimolecular. Unlike SN2’s direct approach, SN1 takes its time. It happens in two steps and forms a carbocation, which is a positively charged carbon that gets all the attention mid-reaction.
- Bond breaks first: The leaving group bids farewell, leaving behind the carbocation.
- Nucleophile attacks: The nucleophile swoops in to fill the carbocation’s empty spot.
This intermediate carbocation is the star. Its stability dictates the entire reaction speed. Tertiary carbocations, which are stabilized by surrounding carbon branches and sometimes resonance, ramp up SN1 rates. Secondary carbocations are moderate, while primary carbocations are usually a no-go for SN1 because they’re too unstable.
And here’s a twist: if the carbocation forms at a chiral center, the nucleophile can attack from either side, producing a racemic mix—think peaceful coexistence of both mirror images.
When is SN1 Favored?
- Bulky tertiary alkyl halides favor SN1, avoiding SN2’s crowd.
- Weak nucleophiles work fine—they don’t have to rush in to attack.
- Polar protic solvents (like water or alcohol) stabilize the carbocation and help the leaving group exit gracefully.
Breaking Down the Chemistry: Who’s Doing What?
Understanding the roles of nucleophiles and electrophiles clears the fog. Nucleophiles have extra electrons—they love to share or give. They usually carry a negative charge or at least a pair of electrons ready to mingle. Electrophiles lack electrons and look for partners. In substitution reactions, electrophiles act like the molecule with the leaving group, while nucleophiles swoop in to swap places.
Think of electrophiles as folks desperate for company and nucleophiles as enthusiastic matchmakers.
Alcohols in the Mix: SN1 vs SN2 Shows Up
Alcohols react differently depending on their structure. Primary alcohols often take the SN2 route when treated with hydrogen halides—nucleophiles attack at once. Secondary and tertiary alcohols prefer the SN1 path, forming carbocation intermediates before the nucleophile joins the party.
This difference fuels how chemists convert alcohols to alkyl halides based on what’s in their toolbox.
Quick Tips for Beginners:
- Look at the carbon center: Primary usually means SN2; tertiary leans SN1.
- Check your nucleophile strength: Strong nucleophiles favor SN2; weak nucleophiles tend to let SN1 take the lead.
- Remember the solvent: Polar protic solvents like water favor SN1; polar aprotic solvents favor SN2.
- Recognize stereochemistry outcomes: SN2 flips the configuration, SN1 forms a mix.
Why Should You Care About These Reactions?
Aside from being a staple in organic chemistry courses, understanding SN1 and SN2 helps make sense of many real-world processes. From drug design to industrial synthesis, these reactions explain how molecules transform gracefully or with flair. Knowing their differences means you can predict reaction behavior, tweak conditions for better yields, or troubleshoot when things don’t go as planned.
In a Nutshell:
SN2: One step, nucleophile and substrate act together, inversion of stereochemistry, best with unhindered molecules.
SN1: Two steps, carbocation intermediate formed, leads to racemic mixture, favored by bulky, stable carbocations and polar protic solvents.
Practical Example: Replacing a Chlorine on an Alkyl Halide
Imagine you have a molecule with a chlorine atom you want to replace with a hydroxide group (–OH). If the carbon attached to chlorine is primary, and you throw in a strong nucleophile like hydroxide ion, the reaction will race through SN2. The hydroxide attacks directly, kicking chlorine out, flipping stereochemistry if any.
If it’s a tertiary carbon, the chloride leaves first, forming a carbocation. Then, hydroxide attacks, and you end up with a racemic mixture if the carbon is chiral. That’s SN1, slow but sure.
Final Thought: Chemistry’s Substitution Switcheroo
Understanding the difference between SN1 and SN2 reactions is like unlocking a secret passage in the labyrinth of organic chemistry. It’s not just about memorizing mechanisms but grasping how molecules choose their paths when the stage is set. Whether you’re a student trying to ace exams or an enthusiast curious about how the chemical world spins, knowing these reactions helps illuminate the dance of atoms.
So next time you think of a molecule swapping guests, ask yourself: does it take the one-step sprint (SN2) or the two-step stroll with a celebrity guest (carbocation) SN1?
The chemistry world awaits your curiosity.
What is the main difference in the reaction steps between SN1 and SN2?
SN1 occurs in two steps, forming a carbocation intermediate first. SN2 happens in a single step where the nucleophile directly replaces the leaving group.
Why does SN1 reaction create a carbocation intermediate?
The leaving group leaves first, breaking the bond to form a positively charged carbocation. This step is slow and controls the reaction speed.
Which types of molecules favor SN1 over SN2 reactions?
SN1 favors tertiary alkyl halides and molecules that can stabilize carbocations. Primary alkyl halides usually undergo SN2 instead.
How does the stereochemistry differ between SN1 and SN2 products?
SN1 produces a racemic mixture due to planar carbocation attack. SN2 causes inversion of configuration at the reaction site.
What factors affect the rate of SN1 reactions?
SN1 rate increases with stable carbocations and polar, non-acidic solvents like alcohols or water. Also, weak bases favor the reaction.



Leave a Comment