E1 or E2? Understanding the Mechanistic Choice
The decision between E1 and E2 elimination mechanisms depends primarily on the nature and strength of the base involved, along with the solvent and substrate environment.
Impact of Base Strength and Charge
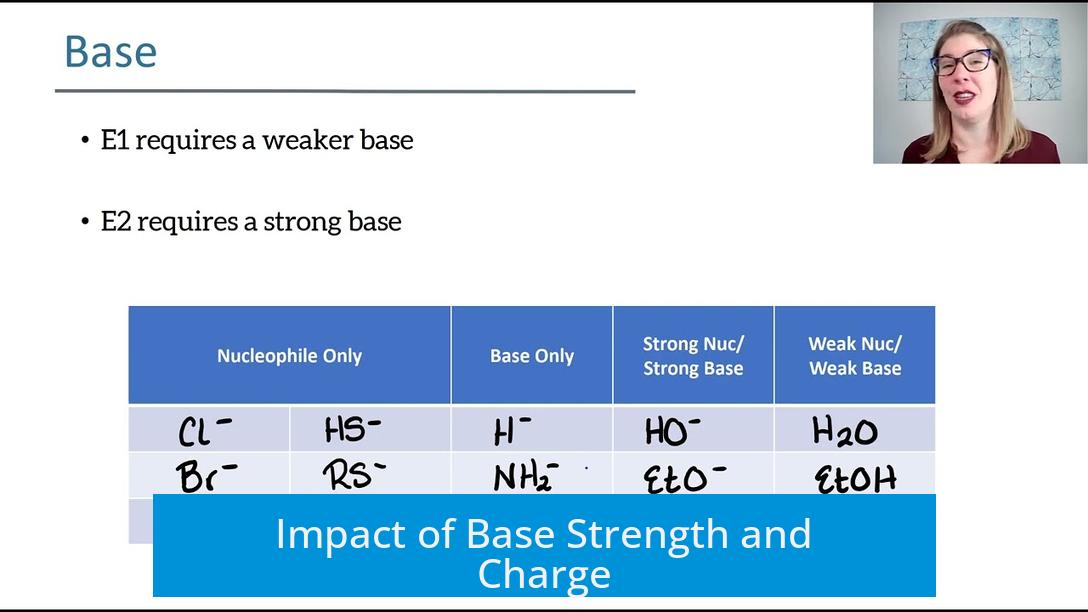
The base determines whether elimination proceeds via E1 or E2. If the base is t-BuO-H (tert-butyl alcohol), the scenario favors E1. This is because t-BuO-H is a very weak, uncharged base. Oxygen’s electronegativity impedes effective lone pair donation, and the conjugate acid formed is relatively unstable. The bulky, weak nucleophilic nature of t-BuO-H fails to abstract a proton efficiently from the substrate, making E1 more probable.
By contrast, using t-BuO− (tert-butoxide ion), often provided as t-BuO-K, leads to E2. This base is strong, negatively charged, and highly reactive. The charged oxygen readily donates its electron pair to remove a β-hydrogen, facilitating a concerted, one-step elimination without carbocation formation.
Solvent and Substrate Effects
E1 reactions occur typically in polar protic solvents. Here, t-BuOH may act as both reactant and solvent. Its bulkiness and weak nucleophilicity do not favor nucleophilic substitution but support carbocation formation. Secondary alkyl halides with good leaving groups in polar protic solvents encourage E1, with solvent stabilizing the carbocation intermediate.
Carbocation Rearrangements and Product Distribution
E1 mechanisms allow carbocation rearrangements such as hydride shifts. When a secondary carbocation forms near a tertiary carbon, a hydride shift stabilizes the intermediate. These rearrangements influence the final product distribution.
The major product in E1 typically is the thermodynamically favored, more substituted alkene. The proton abstraction occurs after carbocation formation and possible rearrangements, yielding the most stable olefin.
Summary Table
| Condition | Base | Mechanism | Key Features | Products |
|---|---|---|---|---|
| Weak, uncharged base | t-BuO-H (tert-butyl alcohol) | E1 | Polar protic solvent, carbocation intermediate, rearrangements possible | More substituted alkene (thermodynamic product) |
| Strong, charged base | t-BuO− (tert-butoxide ion) | E2 | One-step proton abstraction, no carbocation intermediate | Less hindered alkene, kinetic control |
Key Takeaways
- Use of t-BuOH promotes E1, due to weak, uncharged base and polar protic environment.
- Use of t-BuO− leads to E2, involving a strong, charged base that abstracts β-hydrogen directly.
- E1 allows carbocation rearrangements, influencing product stability and distribution.
- The major product from E1 is typically the most substituted, stable alkene.
What determines if a reaction follows E1 or E2 when using tert-butyl compounds?
The nature of the base is key. t-BuO-H is weak and uncharged, favoring E1. t-BuO⁻, a strong charged base, favors E2 by rapidly removing protons.
How does the solvent affect whether an E1 or E2 mechanism occurs?
Weak bases and polar protic solvents, like t-BuOH, support E1. They stabilize carbocations and slow direct proton removal needed for E2.
Can carbocation rearrangement happen in E1 mechanisms?
Yes, carbocations can rearrange via hydride shifts in E1. This stabilizes intermediates and influences the final product structure.
Why is the tert-butoxide ion more likely to cause E2 than tert-butyl alcohol?
Because t-BuO⁻ is charged and strongly basic, it rapidly removes beta hydrogens leading to concerted E2 elimination. t-BuOH is weak and favors stepwise E1.
What kind of products form from E1 reactions with tertiary substrates?
E1 typically produces the more substituted, stable alkene. The product distribution favors thermodynamic stability after carbocation formation.





Leave a Comment