Endo vs Exo Product: One Substituent on Dienophile
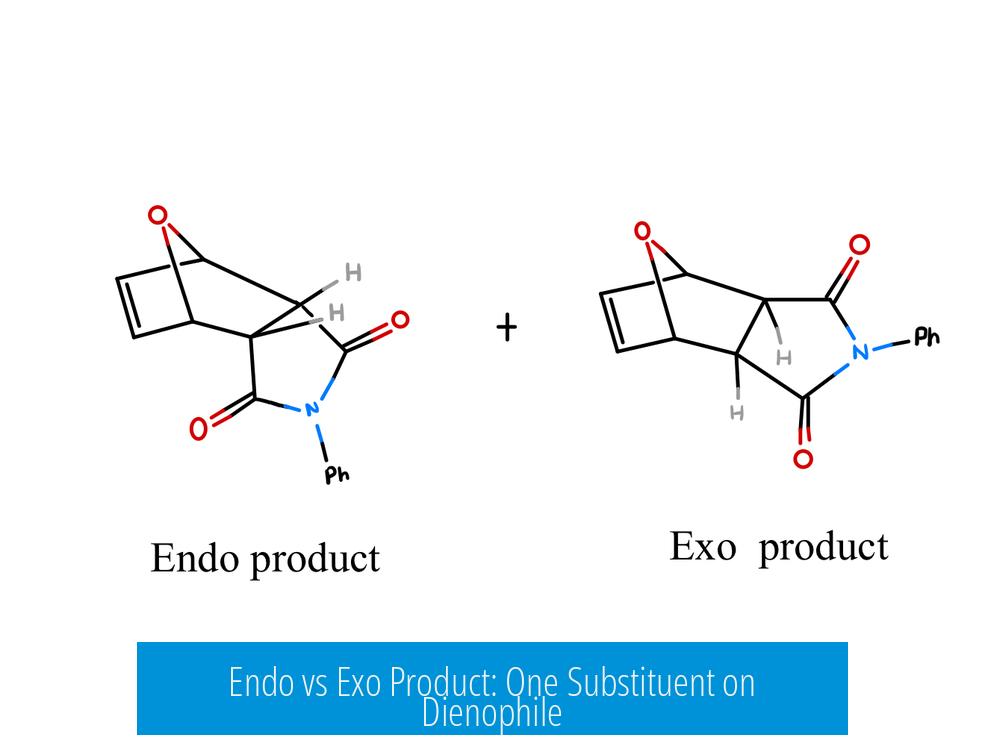
The difference between endo and exo products in a Diels-Alder reaction with one substituent on the dienophile depends on the relative orientation of that substituent to the newly formed bridge in the bicyclic system. The endo product places the substituent under (or syn to) the bridge, while the exo product positions it away (anti to) the bridge.
Identifying Exo Products
Exo products result when the substituent on the dienophile points in the same direction as the bridge of the bicyclic system. This orientation is often revealed by drawing the transition state mechanism. An exo product typically has the substituent positioned away from the newly formed bicyclic ring’s face.
Stereochemical Outcomes and Racemic Mixtures
When a dienophile such as acrolein participates, it lacks regioisomers because its substituent pattern is simple. However, the diene and dienophile can approach from either face, leading to stereoisomers. This face selectivity frequently causes a racemic mixture, which is an equal mix of enantiomers.
Regioselectivity and Resonance Considerations
Correct prediction of endo or exo products requires understanding regioselectivity, which hinges on resonance and charge distribution:
- Examine resonance structures of the diene to locate partial negative and positive charges.
- Analyze resonance in the dienophile to identify complementary partial charges.
- Align the diene and dienophile so that these charges are opposite, favoring interaction and correct regioisomer formation.
This alignment influences both regioselectivity and stereoselectivity, guiding toward the preferred product.
Structural Orientation and Its Effect

The exo product is characterized by the substituent (e.g., an aldehyde in acrolein) pointing in the same direction as the bicyclic bridge, such as a CH2-N bridge in some systems. This stereochemical placement contrasts with the endo approach, where the substituent points beneath the bridge, often favored by secondary orbital interactions.
| Product Type | Substituent Orientation | Relation to Bridge |
|---|---|---|
| Endo | Under or syn | Close to (beneath) bridge |
| Exo | Over or anti | Away from bridge |
Key Takeaways
- Endo/exo differ by substituent orientation relative to bicyclic bridge.
- Exo identified when substituent points in same direction as bridge.
- Stereochemistry results in racemic mixtures due to approach from two faces.
- Regioselectivity depends on resonance and charge alignment.
- Transition state analysis clarifies endo vs. exo configuration.
Endo vs Exo Product (One substituent on dienophile): Unlocking the Mystery of Stereochemistry
When it comes to distinguishing the endo and exo products in a Diels–Alder reaction with one substituent on the dienophile, the key lies in the spatial orientation of that substituent relative to the bicyclic bridge. Put simply: the exo product is the one where the substituent points away from the forming bridge, and the endo product has it pointing toward it. But don’t stop there—there’s more nuance that makes this topic both scientifically intriguing and practically relevant.
Let’s unwrap this molecular puzzle step-by-step, highlighting how resonance, transition states, and stereochemical approaches determine the final arrangement. And yes, we’ll throw in some fun along the way to keep you engaged (because chemistry need not be dry!).
Recognizing the Exo Product – The Visual Clue
Have you ever looked at a product and wondered, “Is this exo or endo?” Thankfully, chemists aren’t left guessing thanks to transition state analysis. The product you drawn as exo is confirmed by studying its formation mechanism. When you sketch out the reaction’s transition state, the exo configuration usually pops out clearly.
Specifically, in our case with one substituent on the dienophile (say, an aldehyde group), the exo product features the aldehyde pointing in the same direction as the CH2-N bridge in the bicyclic system. This structural orientation is pivotal—picture a subtle molecular “handshake” where the substituent and the bridge align on the same side, making the exo the distinct “face” of the product.
Why Does This Stereochemistry Matter?
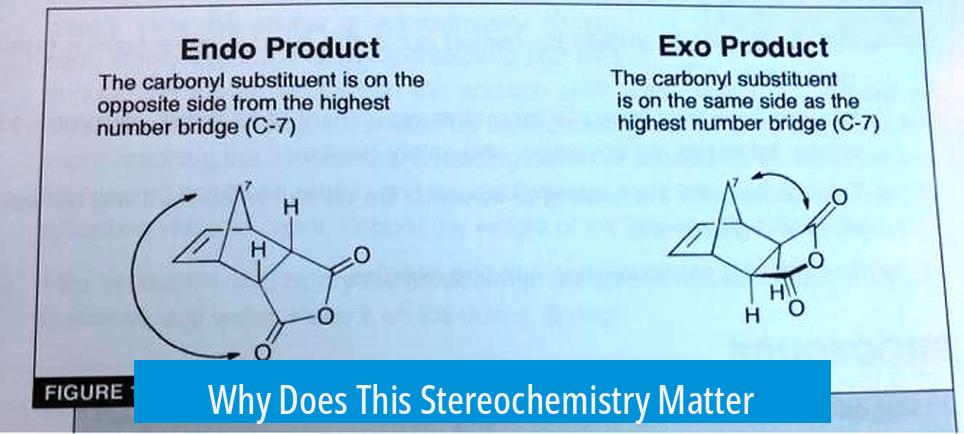
The difference between endo and exo isn’t just a trivial classification; it impacts the physical, chemical, and biological properties of the product. For example, the reaction’s selectivity influences yield, reactivity, and even enzyme interactions if we venture into biological systems.
Advanced applications, like designing pharmaceuticals or natural product syntheses, demand precise control over this stereochemistry. Knowing how to predict and verify whether your product will be endo or exo saves tons of time (and possibly expensive reagents) in the lab. You want your reaction making the right product the first time, right?
The Sneaky Role of Resonance in Regioselectivity
Now, here’s a twist: While we focus on the endo/exo distinction, it’s crucial to remember that regioisomers lurk in the background. Take acrolein as your dienophile—it has no regioisomers on its own. But the limit is that the diene and dienophile can approach from two different faces, resulting in a racemic mixture rather than a single stereoisomer.
Before declaring victory on the product label, it’s wise to analyze the resonance structures of both the diene and the dienophile. These resonance contributors reveal where partial positive and negative charges hover. Aligning these opposite charges during the cycloaddition ensures that the reaction proceeds with the best regioselectivity.
Imagine the diene’s negative charge happily meeting the dienophile’s positive charge. This alignment is like a well-matched dance — making sure the “partners” come together in the right way to decide who ends up where on the molecular dance floor.
Practical Tips for Predicting the Right Product
- Draw the resonance forms: For the diene, sketch resonance contributors to locate partial negative regions; for the dienophile, find partial positives.
- Align charges: Orient the molecules so that opposing partial charges face each other. If not, your predicted product might be off by more than just a little tilt.
- Check the transition state: Look at the bicyclic framework forming. If the substituent sits on the same side as the bridge (like your CH2-N), you’re likely looking at an exo product.
These steps improve your predictive powers beyond guesswork. In fact, chemistry becomes a bit like crime scene investigation. You pick up clues from resonance, transition states, and substituent positions to solve the stereochemical mystery.
The Endo vs Exo Debate: Why Does Nature (and Chemists) Often Prefer Endo?
Funny thing is, despite the clarity of exo product identification, reality often shows the reaction favoring endo products due to the famous endo rule, which involves secondary orbital interactions stabilizing the transition state. But the exo product *can* be the major one, especially when bulkier substituents or experimental conditions favor its formation.
So, when one substituent is present on the dienophile, like in our acrolein example, the stereochemical outcome depends heavily on transition state geometry and substituent orientation. Does the aldehyde group want to cozy up to the bridge? If yes, endo might win. If no, and it points the other way, the exo product claims the spotlight.
Racemic Mixtures: The Double Trouble

Because the diene and dienophile can approach from either face, two enantiomeric products often form in equal amounts, making your product a racemic mixture. This mixture can be tricky if you want just one “handedness” of the molecule. Techniques like chiral catalysts or asymmetric synthesis come into play here, but that’s a story for another blog.
In Summary: What You Need to Remember About Endo vs Exo with One Substituent on Dienophile
- Exo products have substituents pointing on the same side as the bridge; transition state analysis confirms this.
- Resonance effects determine regioselectivity; always map partial charges before concluding on product structure.
- Approach of diene and dienophile can generate racemic mixtures, complicating stereochemistry.
- Substituent orientation in the transition state dictates endo vs exo, influencing reaction outcomes.
So, next time you’re faced with the dilemma “Is my product endo or exo?” arm yourself with resonance drawings, check your transition states, and consider stereochemical approaches. Your molecules will thank you by forming predictably and neatly—no awkward surprises in your flask!
And hey, if you still find this dizzying, remember: even top chemists double-check their drawings and mechanisms. Chemistry is as much art as it is science, and every stereochemical twist is a new plot twist waiting to be unraveled.
What distinguishes the endo and exo products when there is one substituent on the dienophile?
The difference lies in the orientation of the substituent in the transition state. In the exo product, the substituent points away from the diene’s bridge, while in the endo, it points toward it.
How can resonance be used to predict the correct product configuration?
Resonance helps locate partial charges on both the diene and dienophile. Aligning opposite charges between them guides regioselectivity and influences whether the endo or exo product forms.
Why does the reaction with acrolein often produce a racemic mixture?
Acrolein has no regioisomers, but the diene and dienophile can approach from either face. This leads to two enantiomers, creating a racemic mixture of products.
How is the exo product identified structurally when the dienophile has an aldehyde substituent?
The exo product is indicated when the aldehyde points in the same direction as the diene’s bridge (e.g., CH2-N bridge). This orientation determines the stereochemical outcome.
What is the role of the transition state in determining the product as endo or exo?
Analyzing the transition state mechanism reveals the substituent’s position. The product you draw matches the conformation in the transition state, identifying it as endo or exo.



Leave a Comment