How to Differentiate Between E1 and E2 Mechanisms for a General Reaction

E1 and E2 mechanisms differ mainly in their sequence of bond-breaking and bond-forming steps, the intermediates involved, and the conditions that favor each. Identifying these distinctions helps predict the mechanism a reaction will follow based on experimental conditions and molecular structure.
1. Nature of the Base and Reaction Conditions
The type of base and the reaction’s environment significantly influence whether a reaction proceeds through an E1 or E2 mechanism.
- E1: Operates under neutral or acidic conditions or in the presence of a weak base. Examples include water and alcohols. The base strength is generally unimportant for E1 since it relies primarily on carbocation formation.
- E2: Requires a strong base to proceed efficiently. Strong bases such as methoxide (MeO−), ethoxide (EtO−), potassium tert-butoxide (t-BuOK), DBN, DBU, or LDA favor E2 eliminations.
2. Carbocation Formation and Stability
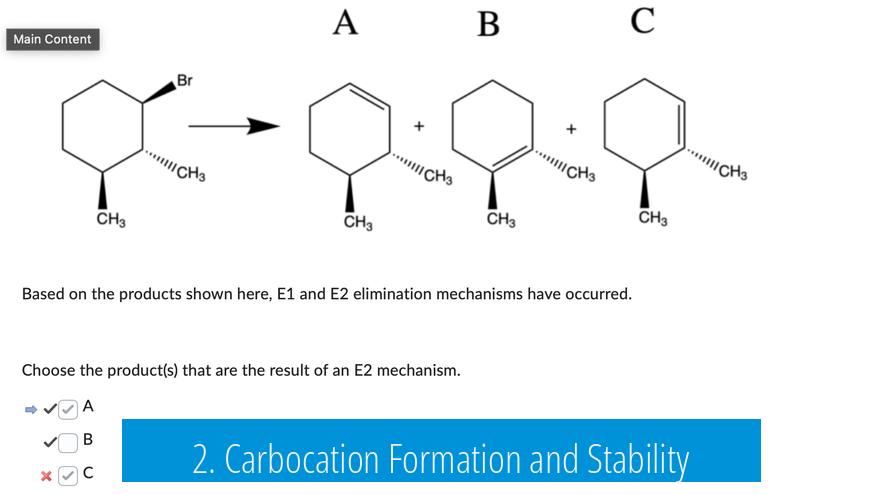
The presence or absence of carbocation intermediates is a defining feature that separates E1 from E2 mechanisms.
- E1: Proceeds via the formation of a carbocation intermediate. This intermediacy means the reaction rate depends solely on the substrate concentration. Primary carbocations are highly unstable, making E1 rare or non-occurring for primary substrates under standard conditions.
- E2: Does not form a carbocation intermediate. Instead, elimination occurs in a single concerted step where the base removes a proton while the leaving group departs simultaneously.
3. Stereochemistry and Product Distribution
Stereochemical outcomes reveal valuable clues about the mechanism behind an elimination reaction.
- E1: The carbocation intermediate is planar, causing loss of stereospecificity. This leads to a mixture of E (trans) and Z (cis) alkene isomers. No spatial arrangement constraints are required during base abstraction.
- E2: Exhibits stereoselectivity due to its requirement of an antiperiplanar arrangement. The β-hydrogen and the leaving group must be positioned 180° apart. This spatial requirement generally produces one predominant E or Z isomer.
4. Reaction Mechanism Steps
| Mechanism | Process |
|---|---|
| E1 | Stepwise: First, the leaving group departs forming a carbocation, then a base removes a proton forming the alkene. |
| E2 | Concerted: The base abstracts a proton as the leaving group departs simultaneously, forming the alkene in one step. |
5. Solvent Effects
Solvents can affect the stability of possible intermediates, thus influencing the favored pathway.
- E1: Favored by polar protic solvents such as water and alcohols that stabilize the carbocation intermediate via solvation. These good ionizing solvents make carbocation formation easier.
- E2: Less affected by solvent polarity since no carbocation intermediate forms. Hence, it can proceed in a wider range of solvents, including polar aprotic ones.
6. Rate of Reaction Dependence
The rate laws of E1 and E2 mechanisms reflect the difference in reaction steps.
- E1: Rate depends only on the concentration of the substrate because carbocation formation is rate-limiting, making it a unimolecular elimination reaction.
- E2: Rate depends on both substrate and base concentrations since the reaction proceeds in a single concerted step involving both species. This makes the reaction second-order overall.
7. Substrate Reactivity Order
Substrate structure influences whether E1 or E2 is more feasible.
- For both mechanisms, tertiary (3°) substrates react faster than secondary (2°), which react faster than primary (1°).
- E1: Typically does not occur with primary substrates due to carbocation instability.
- E2: Occurs with primary, secondary, and tertiary substrates, especially with strong bases.
Summary Table: Key Differences Between E1 and E2
| Characteristic | E1 | E2 |
|---|---|---|
| Base Strength | Weak or neutral | Strong |
| Mechanism Steps | Stepwise with carbocation intermediate | Concerted single step |
| Carbocation Intermediate | Yes, rate-determining step | No |
| Stereochemistry | Loss of stereospecificity; mixture of E/Z | Stereospecific; usually antiperiplanar arrangement |
| Solvent Effect | Favored by polar protic solvents | Less sensitive to solvent |
| Reaction Rate | Depends on substrate only | Depends on substrate and base |
| Substrate Preference | Tertiary > Secondary; rarely primary | Tertiary, secondary, primary with strong base |
Practical Examples to Differentiate Mechanisms
When analyzing an elimination reaction, consider the following factors:
- If the base is weak (e.g., H2O, ROH) and the substrate is tertiary or secondary, suspect E1.
- If the base is strong (e.g., t-BuOK, LDA), E2 is likely.
- Observation of rearranged products indicates a carbocation intermediate, confirming E1.
- If the product is a stereoselective alkene (usually single E or Z isomer), the reaction is probably E2.
- Rate studies that show dependence on both substrate and base concentration point to E2, while dependence only on substrate indicates E1.
Additional Notes on Mechanistic Transitions
Sometimes a reaction exhibits mixed features or intermediate behavior depending on conditions:
- With moderate base strength and polar protic solvents, elimination can switch from E2 to a borderline E1cB mechanism.
- Temperature variations can shift competition between E1/E2 and substitution pathways.
- Strong bulky bases tend to favor E2 by blocking substitution and favoring deprotonation.
Key Takeaways
- E1 mechanisms feature a carbocation intermediate and stepwise mechanism favored by weak bases and polar protic solvents.
- E2 mechanisms proceed in one concerted step with strong bases and require an antiperiplanar arrangement for elimination.
- The rate of E1 depends only on substrate concentration; E2 depends on both substrate and base.
- Stereochemical outcome helps identify mechanism: mixtures of E/Z alkene in E1 versus stereoselective products in E2.
- Primary substrates generally do not undergo E1 due to carbocation instability, but E2 can proceed with strong bases.
How Can You Differentiate Between E1 and E2 Mechanisms for a General Equation of a Reaction?
Let’s cut to the chase: the key to distinguishing between E1 and E2 mechanisms lies in understanding the nature of their reaction pathways, the role of the base, carbocation formation, stereochemistry, and reaction rates. Sounds like chemistry class in superhero mode, right? But stick with me — it’s not only doable but also useful and kinda fun!
Imagine you’re at the chemistry crossroads, and you want to know if your elimination reaction is taking the smooth, one-step highway (E2) or the scenic, two-step route (E1).
1. The Base Plays the Role of “Reaction Boss”
The very first clue is the strength of your base. E1 reactions hang out with weak bases or even neutral and acidic conditions. Think of bases like water or alcohols—these are chill, easy-going, and quite happy to let the other party do the job.
In contrast, E2 screams “strong base”! They require a meal packed with potassium tert-butoxide, DBN, LDA, or ethoxide. These heavy hitters don’t wait around; they push for elimination as fast as possible.
If your reaction is using a wimpy base, suspect E1. If it’s rocking the strong bases, you’re likely in E2 territory. Simple rule but surprisingly powerful.
2. Carbocation Formation: The Drama Queen of the Reaction
This is where the plot thickens. E1 mechanisms love drama: a carbocation intermediate forms after the leaving group drops out, creating a positively charged species that waits before the proton leaves.
However, let’s be clear—primary carbocations are the bad actors who rarely show up because they are too unstable. It’s like inviting a fire hazard to the party. So primary substrates usually don’t follow E1 mechanics.
E2 mechanisms are no-nonsense; they skip the carbocation step entirely, performing a concerted one-step elimination where the base pulls a proton exactly as the leaving group exits. No waiting around for intermediates—just straight action.
3. Stereochemistry: The Shape of the Ending
Want to peek into the reaction’s secrets? Look at your product’s stereochemistry.
E1 loves to twirl and spin: since the carbocation intermediate is planar, the proton can be removed from either side, leading to a mixture of E and Z isomers. No strict rules here. The product loses stereospecificity.
E2, however, is more like a disciplined dancer. It follows the “antiperiplanar” rule, requiring the hydrogen and the leaving group to be opposite each other in the molecule’s plane. This leads to stereoselective products, often just one major E or Z isomer.
Seeing one clear isomer? You might be watching an E2 show. Seeing a confusing blend of isomers? That’s an E1 fiesta.
4. Reaction Speed and Dependency: Who’s in the Driver’s Seat?
An easy way to recall this is that E2 is a bimolecular process. The rate depends on both the substrate and the base concentration. Strong base + substrate = faster rate.
E1 is unimolecular, meaning the rate depends only on the substrate, not the base. This reflects its two-step mechanism — first forming the carbocation, which is the slow step.
5. Solvent and Reaction Conditions: The Environmental Clues
The neighborhood matters! E1 reactions thrive in polar protic solvents like water or alcohols, which stabilize the carbocation intermediate and encourage ionization.
E2 doesn’t particularly need special solvent behavior—it’s more base-strong and direct.
6. Rearrangements: Plot Twists in the Mechanism
Since E1 forms carbocations, the reaction can rearrange (hydride or alkyl shifts) to create more stable carbocations, resulting in different product profiles.
E2 has no time for such drama—no intermediates, no rearrangements.
Example: You have a reaction with 2-bromo-2-methylbutane. You treat it with methanol (a weak base). The elimination proceeds slowly with a mixture of E/Z alkenes, and some rearranged products appear. All signs point to E1.
Now, switch to potassium tert-butoxide (a strong base) and the reaction becomes fast, clean, and gives mainly the more substituted alkene with a consistent stereochemistry—E2 in action.
The Bottom Line
Try asking yourself a few straightforward questions to nail down the mechanism:
- How strong is the base?
- Is the reaction rate dependent on base concentration?
- Are carbocation rearrangements observed?
- Is the product stereospecific or a mixture?
- What solvent is being used?
When the base is strong, the rate depends on both base and substrate, the elimination proceeds in one step without rearrangement, and the product is stereospecific — welcome to E2 territory.
When the conditions favor carbocation formation, rearrangements occur, the reaction is unimolecular in rate, and products include E/Z mixtures, E1 is your answer.
Why Does It Matter?
Understanding which elimination path a reaction follows helps chemists predict product distribution, optimize reaction conditions, and avoid undesired by-products. It also informs synthesis strategies that require control over stereochemistry, like making specific drugs or materials.
So next time you stare down an elimination reaction, remember: don’t just follow the recipe blindly—peek behind the curtain and watch the mechanism unfold. After all, chemistry is as much about the journey as it is about the product.
What base strength favors E1 versus E2 mechanisms?
E1 occurs with weak or neutral bases, like water or alcohols. E2 needs strong bases such as ethoxide or tert-butoxide. The choice of base greatly influences which mechanism will take place.
How does carbocation formation help distinguish E1 from E2?
E1 involves a carbocation intermediate, leading to possible rearrangements. E2 does not form a carbocation, as it is a single-step concerted reaction. Presence of carbocation intermediates points to E1.
What stereochemical differences indicate E1 or E2 mechanisms?
E1 produces a mix of E and Z alkene isomers due to planar carbocation intermediates. E2 shows stereoselectivity, forming primarily one isomer because elimination requires an antiperiplanar geometry.
How do reaction steps differ between E1 and E2?
E1 is a two-step mechanism with carbocation formation followed by elimination. E2 is a one-step process where proton removal and leaving group departure happen simultaneously.
How does solvent choice affect E1 and E2?
E1 is favored by polar protic solvents that stabilize carbocations. E2 generally occurs in less polar or polar aprotic solvents that support strong bases and concerted elimination.




Leave a Comment