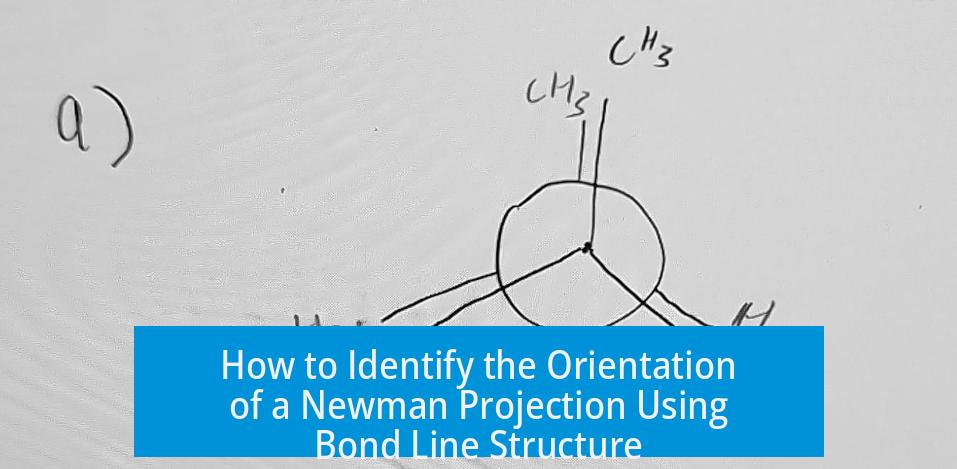
How to Determine if a Newman Projection is Right Side Up or Upside-Down Y from a Bond Line Structure
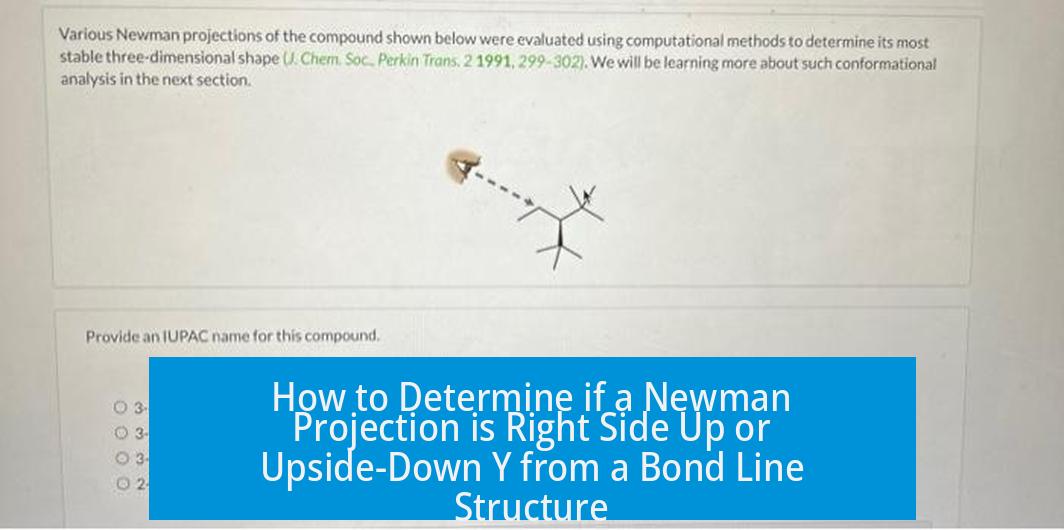
Newman projections do not have a fixed “right side up” or “upside-down Y” orientation based on bond line structures because the molecule can be rotated in space without changing its identity. The orientation you choose to draw the Newman projection is arbitrary and does not affect the molecular properties.
Why Orientation Does Not Matter
Newman projections visualize the molecule as seen along a specific bond axis. When you rotate or flip the molecule, you effectively change the perspective. Drawing the projection inverted or flipped simply represents the same molecule from a different angle.
- Rotating the molecule 180° changes the projection orientation but not the structure itself.
- Flipping the structure upside-down results in an inverted Newman projection.
- The chemical identity and conformational energies remain unchanged despite orientation.
Atom Arrangement and Chirality Influence Interpretation
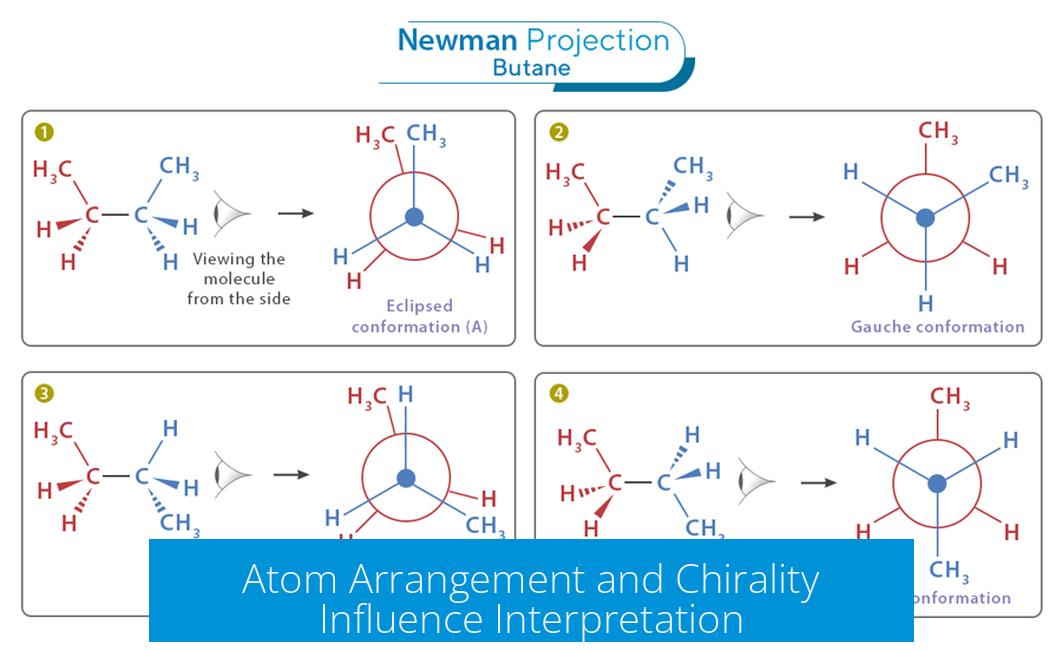
Though the projection’s “up” or “down” is irrelevant, the relative positioning of substituents is critical. The order of atoms attached to the front and back carbons governs molecular chirality and conformations.
- Arrange substituents consistently to accurately compare conformations.
- Identify which groups are eclipsed or staggered for energy analysis.
- Chiral centers depend on this spatial order of atoms.
Practical Tips for Drawing Newman Projections from Bond Lines
https://www.youtube.com/watch?v=wgBzWgDdPYM
- Identify the bond axis you want to view along in the bond line structure.
- Visualize looking along that bond, placing front carbon substituents closer in the projection.
- Position back carbon substituents behind the front carbon in the Newman view.
- Do not worry about keeping the projection “right side up”; focus on relative substituent positions.
Conclusion

Determining if a Newman projection is “right side up” or “upside-down Y” is a matter of perspective, not a strict rule based on bond line structure. The key is to maintain the correct spatial order of substituents around the carbon atoms to represent the molecule’s true conformation and stereochemistry accurately.
- Newman projection orientation is arbitrary and flexible.
- Rotation or flipping does not change molecular identity.
- Substituent arrangement and chirality drive conformational insight.
- Focus on relative positions, not diagram orientation, for clarity.
How to Determine if a Newman Projection is a Right Side Up or Upside-Down Y Based on the Bond Line Structure
Wondering about the mysterious “right side up” or “upside-down Y” in Newman projections? It sounds complicated, but here’s the secret: the orientation of the Newman projection itself—whether you see it as right side up or flipped—does not change the molecule’s identity. Let’s unpack exactly what this means and why it matters for your chemistry journey.
Imagine holding a model of a molecule and turning it upside down. The atoms don’t change; they’re just viewed from a different angle. Same idea applies to Newman projections. You can draw that “Y” shape either upright or inverted, and it still represents the same conformation. So, no panic if your projection looks like it’s doing a headstand.
Bond Line Structure: The Starting Point
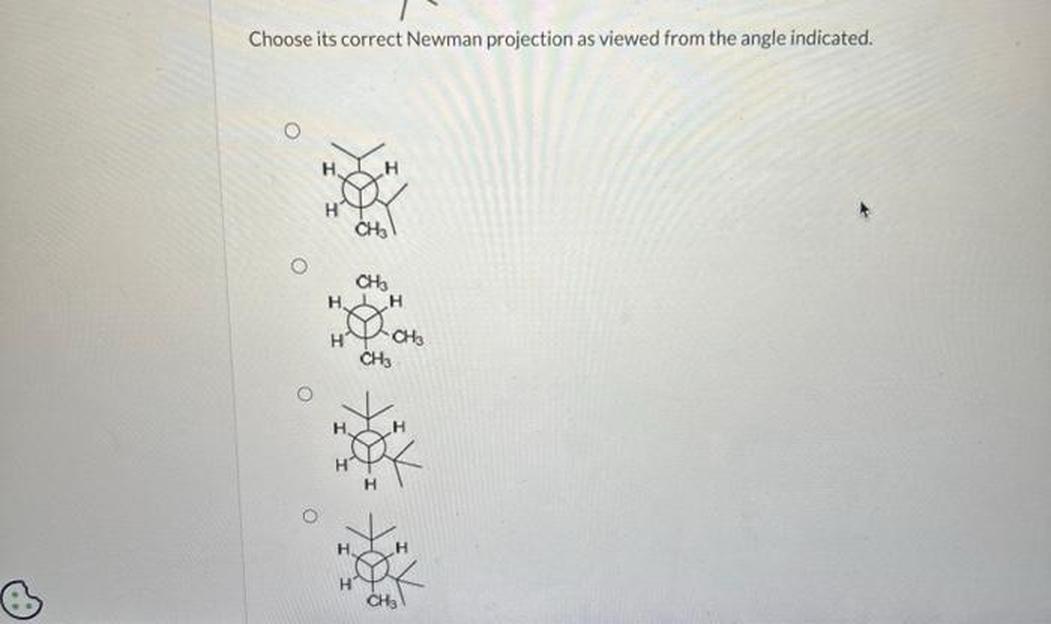
Before diving into the Newman projection, focus on the bond line structure. This structure shows how atoms connect in 2D on paper, representing 3D reality with clever shortcuts. When you look down a bond (say between two central carbons), you’re essentially peeking along a tunnel between these carbons, trying to visualize the spatial arrangement of the attached groups.
The first step? Identify the bond you’re “looking down.” The front carbon is closest to you; the back carbon sits behind it. Think of it as a tiny spyglass view. This sets the stage for your Newman projection.
The Shape of the Y: Up or Down? Does It Even Matter?
The signature Y shape in Newman projections crystallizes when you imagine the three groups attached to the front carbon as the spokes of a wheel centered on that carbon, viewed head-on. The back carbon’s groups form another set of points projected behind these spokes. Naturally, when you sketch this Y, you might draw it right side up, or if you flipped the paper, upside down.
Here’s the kicker: it doesn’t matter. The molecule being the same means your flipped Y means the same thing chemically. Flipping the diagram is like looking at your reflection; it doesn’t alter the underlying reality. Both ways reflect the same arrangement, the same molecule, the same chemistry.
So, How Do You Know Which Way to Draw It Then?

The answer lies not in deciding if the Y is right side up or upside down but in maintaining the relative positioning and order of atoms attached to both carbons. This relationship is crucial to assess the molecule’s stereochemistry and energy states.
Focus on the bonds attached to the front carbon. The three substituents you see must correspond to the actual groups attached in the bond line structure at the front carbon. The same goes for the back carbon. The relative position around each carbon must mirror the real 3D arrangement.
Why? Because this order directly impacts chirality and conformational energy. Some positions create more strain and higher energy; others are more relaxed and stable. So, picking the right orientation to reflect the true substituent order is essential for accurate analysis—even if your Y ends up upside down on paper.
Getting Practical: Tips to Avoid Confusion
- Look directly along the bond axis you want to analyze. Visualize or sketch the front carbon as a dot, with three bonds radiating out at 120° angles.
- Assign groups to each bond based on the bond line structure. Use wedge and dash notation if available to help picture 3D positions.
- Check the back carbon’s substituents similarly. The back carbon is drawn as a circle with three groups attached.
- If flipping or rotating your sketch on paper makes the Y upside down, don’t stress. Just confirm the relative positions are maintained correctly.
For example, if the front carbon has substituents A, B, and C, arranged clockwise in the bond line structure, your Newman projection should show these three groups in the same clockwise order around the front carbon’s center dot. The same logic applies to substituents on the back carbon. This consistency keeps your stereochemistry and conformational analysis spot-on.
Chirality and Energy: Why Details Affect Interpretation
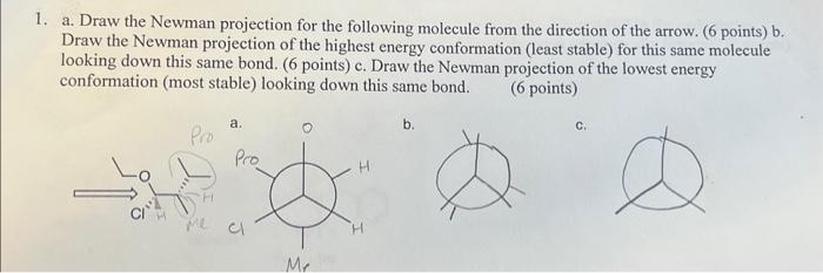
Chirality arises from the spatial arrangement of atoms—mirror images that just can’t align perfectly. When you peek through a Newman projection, preserving the correct order of atoms tells you if you’re looking at an R- or S- configuration or if the conformation is staggered (lower energy) or eclipsed (higher energy).
This nuanced detail shows that deciding “right side up” vs “upside-down” can’t be just visual—it’s chemical. You must identify how substituents relate to one another and observe the bond angles and steric hindrance. Flipping the projection doesn’t change energy or chirality, but misrepresenting which groups attach where will.
Wrapping Up: Why Your Newman Projection Orientation Is Flexible, But Your Atom Order Isn’t
In summary, the orientation of your Newman projection—right side up or upside-down—all depends on your viewpoint and is not chemically meaningful by itself. What truly counts is keeping track of the relative position of each substituent attached to the front and back carbons. This brings accuracy to conformational analysis, chirality assessment, and energy estimation.
Next time you sketch a Newman projection and wonder if your Y looks funny, remember this: it’s like looking at a photo from a different angle. The molecule is the same. Focus instead on correctly pairing substituents from the bond line structure to your Newman drawing. That’s where the chemistry magic really happens.
Got your own tips or tricky experiences interpreting Newman projections? Feel free to share! Sometimes the best learning comes from tales of humble mistakes and aha moments.
How can you tell if a Newman projection is right side up or upside-down based on bond line structures?
Newman projections don’t have a fixed “up” or “down.” Rotating or flipping the molecule gives the same projection meaning. The orientation is flexible and depends on how you view the molecule.
Does flipping the molecule change the Newman projection’s meaning?
No, flipping or rotating the molecule does not change what the Newman projection represents. It’s simply a different perspective of the same molecule.
What role does the arrangement of atoms have in determining a Newman projection’s orientation?
The atom order seen in the Newman projection is crucial. It affects chirality and shows which conformations are more stable or less stable, regardless of whether the projection appears right side up or upside-down.
Is there a “correct” orientation for drawing Newman projections?
There is no single correct orientation. The projection should reflect the relationship between atoms accurately. Both right side up or upside-down Y shapes are valid.
How does chirality affect the interpretation of Newman projections?
Chirality depends on the specific arrangement of atoms around carbons. This arrangement, seen in the projection, influences molecular properties like energy levels.




Leave a Comment