How to Determine Where Hydrogen Goes in Lewis Structures
Hydrogen is placed at the outer positions in Lewis structures because it can form only one covalent bond and never acts as the central atom. This placement aligns with hydrogen’s valence electron count and bonding capacity, which always involves sharing a single pair of electrons.
Valence and Bonding Capacity of Hydrogen
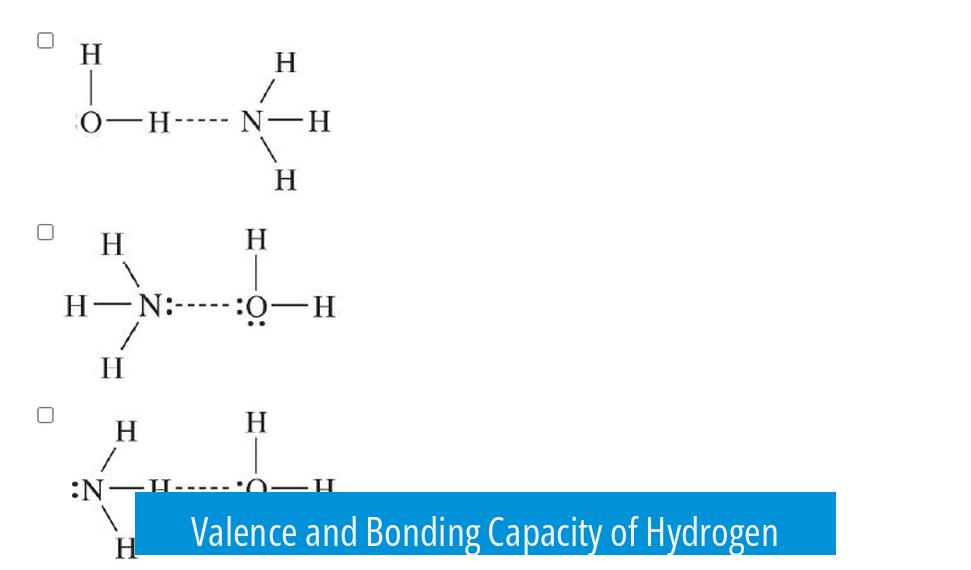
- Hydrogen has 1 valence electron and seeks 2 electrons to fill its outer shell (similar to helium’s electron configuration).
- It can form exactly one covalent bond.
- Once bonded, hydrogen completes its shell and does not form additional bonds.
Consequently, hydrogen always attaches to other atoms rather than serving as a central atom.
General Rule for Hydrogen Placement
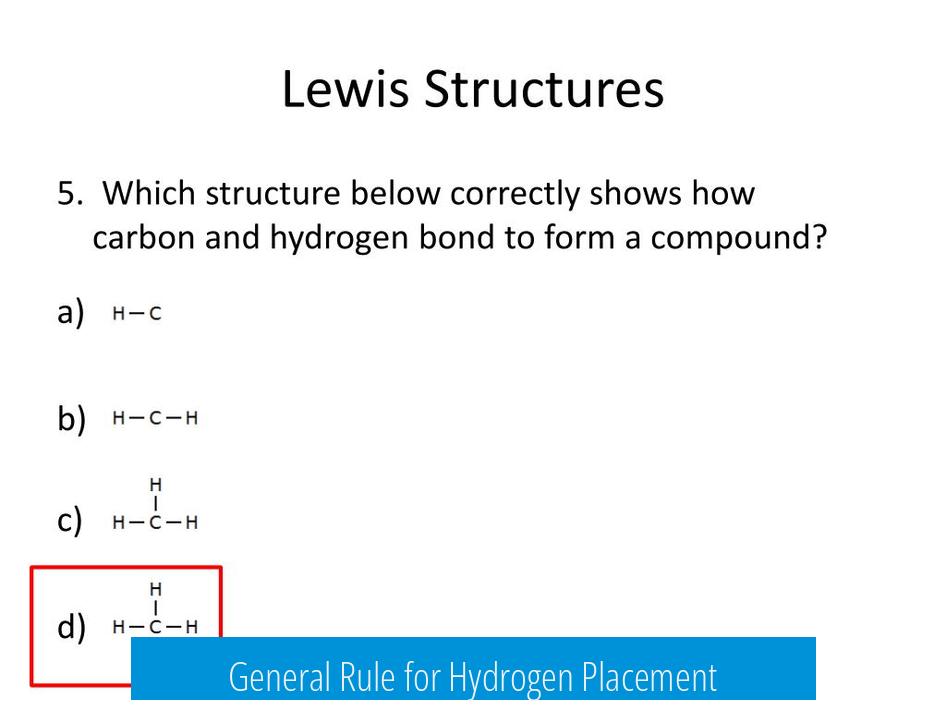
In Lewis structures, the least electronegative atom typically forms the central atom, while hydrogen atoms consistently occupy terminal (outer) positions. This is because hydrogen cannot accommodate more than one bond.
Placement in Inorganic Acids and Oxoanions

- In oxyacids (like HClO3, H2SO4, HNO3), hydrogen atoms attach to oxygen atoms, reflecting the acidic hydrogen’s position.
- Knowing common polyatomic ion and oxoanion formulas helps predict where hydrogen bonds in acid molecules.
Placement in Organic Molecules
- Organic compounds follow different rules where hydrogen attachment shows connectivity and molecular structure.
- For example, in methane (CH4), four hydrogens bond directly to carbon atoms around the central carbon.
- In HCNO, hydrogen bonds to carbon, while the order of elements conveys structural information (not usually the central atom rule).
Examples of Hydrogen Placement
| Molecule | Hydrogen Placement | Reason |
|---|---|---|
| Methane (CH4) | Hydrogens bond to carbon atom (central atom) | Carbon forms 4 bonds; hydrogens form 1 |
| Ammonia (NH3) | Hydrogens bond to nitrogen (central atom) | Nitrogen’s 5 valence electrons accommodate 3 hydrogens |
| HClO3 (Chloric acid) | Hydrogen bonded to oxygen atom(s) | Hydrogen is acidic and attached to oxygen in oxyacids |
| C2H6O (Ethanol and Dimethyl ether) | Hydrogens bonded to carbon or oxygen depending on isomer | Structure reflects connectivity and compound specificity |
Key Takeaways
- Hydrogen forms only one covalent bond; always placed at molecule edges.
- Central atoms are usually less electronegative elements, never hydrogen.
- In oxyacids, hydrogen bonds to oxygen, reflecting acidic hydrogen placement.
- In organic molecules, hydrogen placement shows atomic connectivity and molecular identity.
- Understanding valence electrons and usual bonding patterns guides hydrogen placement.
How Do You Know Where the Hydrogen Goes in Lewis Structures? Breaking Down the Mystery
Here’s the quick and straightforward answer: Hydrogen always goes at the outside of Lewis structures and forms just one bond, because it can only share two electrons to satisfy its duet rule. Yes, it sounds simple, but as soon as molecules get more complex, placing hydrogen correctly demands more attention to bonding rules, atom valence, and molecule specifics.
Let’s unravel this step-by-step with some juicy chemistry facts, real examples, and solid tips. This way, whether you’re sketching simple methane or puzzling over oxyacids, you’ll nail where hydrogen belongs every time.
Valence and Bonding Capacity: The Chemistry ‘Rulebook’ for Hydrogen Placement
Imagine atoms lined up in a dance. Each atom’s moves are defined by its valence electrons—the dots in Lewis structures. Hydrogen shows up with one valence electron and only needs to “hold hands” with one partner to fulfill its duet rule. No fancy backup moves.
Unlike carbon or oxygen, which want eight electrons (the octet), hydrogen’s satisfied with two—just like the noble gas helium. It forms exactly one covalent bond. That’s it.
So when you place hydrogen in a Lewis structure, think simple: hydrogen can’t be the center of the molecule because those atoms like carbon, nitrogen, or oxygen usually act as hubs. Hydrogen is the guest hanging on the fringe.
General Rule for Lewis Structures: Hydrogen Is Always on the Outside
When sketching out any molecule, *put the least electronegative atom in the middle and hydrogen on the outside.* Electronegativity means how much an atom “pulls” electrons toward itself.
Hydrogen is too shy to sit at the center. It can’t handle bonding to more than one atom—put it on the edge, attached to more electronegative atoms like oxygen, nitrogen, or carbon.
For example, in ammonia (NH3), hydrogen atoms hug the nitrogen atom, not the other way around. Nitrogen is the middleman holding the molecular framework.
Oxyacids and Oxoanions: Where Hydrogen Loves to Attach
Things get a little trickier with inorganic acids like HClO3 (chloric acid). Here’s the key: in oxyacids, all hydrogens are attached to oxygens, not to chlorine or other central atoms.
Why? The acidic hydrogen is typically bonded to oxygen in these oxoanions. This hydrogen can dissociate as H+ (a proton), which is what makes them acids. Recognizing oxoanion formulas lets you place hydrogens correctly every time.
Think of it as knowing the family tree. If you know where the oxygen-rich branches are, you know where the hydrogens will cling.
Organic Compounds: Hydrogen Placement Shows More Than Just Location
In organic chemistry, hydrogen placement becomes even more meaningful. Take HCNO, an organic molecule; putting hydrogen by carbon versus nitrogen changes everything.
For example, HCNO isn’t the same as HNCO (isocyanic acid). The position of hydrogen conveys *connectivity* and *specificity*, determining the molecule’s identity and properties.
This means hydrogen’s position isn’t just about following bonding rules, but also about revealing the unique story within the molecule’s formula.
Examples Light the Way
- Methane (CH4): Four hydrogen atoms symmetrically attached to a lone carbon atom. Carbon is the central atom because it can form four bonds. Hydrogens cluster on the outside, no exceptions here.
- Ammonia (NH3): Nitrogen in the center makes three single bonds to three hydrogens. Nitrogen holds some lone pair electrons, and hydrogen is still an outsider guest.
- Ethanol and Dimethyl Ether (C2H6O): Both have the same molecular formula but different Lewis structures. Ethanol attaches some hydrogens to carbon and one to oxygen (alcohol group), while dimethyl ether attaches hydrogens only to carbons; oxygen acts as a bridge. The hydrogen placement here shows the bonding pattern and determines the molecule’s identity.
Putting It All Together: Practical Tips for Knowing Where Hydrogen Goes
- Identify the central atom: Usually the least electronegative (except hydrogen—never central).
- Count valence electrons: Know hydrogen has one and makes one bond.
- Draw the skeleton: Start by attaching hydrogens to atoms that generally bond with them (carbons, oxygens, nitrogens).
- Consider special cases: For oxyacids, hydrogens attach to oxygen atoms, not the central atom, because acidic protons come from oxygens.
- Use molecular formulas wisely: Different formulas (e.g., HCNO vs. HNCO) can give two very different compounds due to hydrogen placement.
- Fulfill bonding rules: Make sure every hydrogen has one bond and that other atoms satisfy octets.
Why Does This Matter? Understanding Hydrogen Placement Unlocks Molecular Secrets
Knowing where hydrogen goes gives vital clues about the compound’s reactivity, acidity, and identity. It helps you predict the behavior of acids, recognize isomers in organic chemistry, and draw accurate molecular models for chemistry exams or research.
For students and chemists alike, mastering this skill turns Lewis structures from a black box into a clear map. No more guessing—just confident placement backed by valence rules and molecular logic.
Have you ever struggled with placing hydrogen in tricky molecules? Now you know hydrogen’s secret: it’s a one-bond, outside-the-central-atom kind of guest. Master that, and you’re halfway home!
So next time you stare at a formula wondering, “Where does hydrogen go?” remember: **hydrogen respects the duet rule, stays on the outside, and loves hanging off atoms like oxygen and carbon.** That’s chemistry you can count on.
How do you determine where to place hydrogen in Lewis structures?
Hydrogen always forms one covalent bond. It is placed where it can form this single bond, often attached to atoms like carbon, oxygen, or nitrogen. In general, hydrogen goes on the outside since it only needs two electrons.
Why does hydrogen never go in the center of a Lewis structure?
Hydrogen can only form one bond and holds just two electrons in its valence shell. The center atom usually has a lower electronegativity and can make multiple bonds. This restricts hydrogen to the outer positions.
Where does hydrogen attach in oxyacids like HClO3?
In oxyacids, hydrogen attaches to oxygen atoms in the oxoanions. For example, in HClO3, hydrogen is bonded to an oxygen, not chlorine. This bonding pattern reflects acidity and structure.
How do Lewis structures differ for organic compounds regarding hydrogen placement?
Organic molecules show hydrogen placement to indicate connectivity. For example, in HCNO, hydrogen bonds to carbon, showing specific structure. Changing hydrogen’s position changes the identity of the compound.
Can you give examples of hydrogen placement in common molecules?
Methane (CH4) has four hydrogens bonded to carbon. Ammonia (NH3) has hydrogens bonded to nitrogen. These examples follow hydrogen’s rule of forming one bond and always being on the outside.



Leave a Comment