Understanding Hydrohalogenation Stereochemistry: Syn and Anti Addition
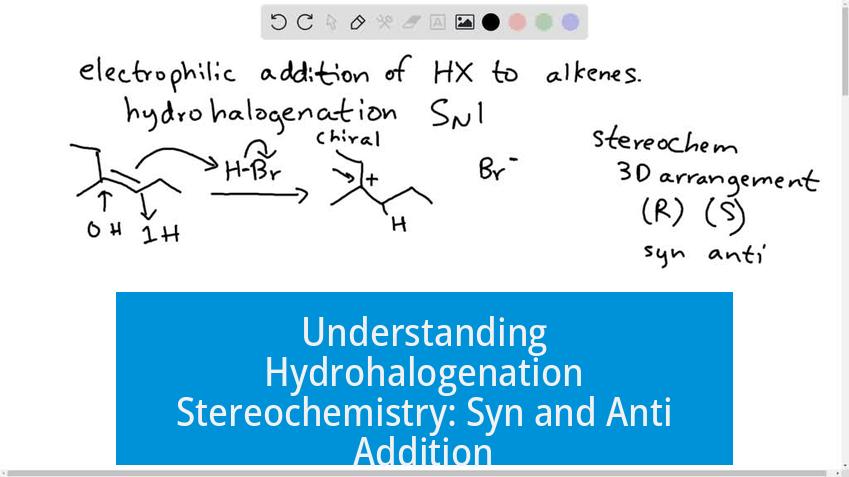
In hydrohalogenation reactions, the syn and anti addition products differ in the spatial arrangement of the added hydrogen and halide atoms across the double bond. Specifically, the two circled products in red are enantiomers resulting from anti addition, where the hydrogen and halogen add to opposite faces of the alkene.
Distinguishing Syn vs. Anti Addition Products
Hydrohalogenation adds hydrogen (H) and a halogen (e.g., Cl) across an alkene’s carbon-carbon double bond. The key to identifying syn or anti addition lies in the relative orientation of these substituents.
- Syn addition: Both H and halide add to the same face (side) of the alkene.
- Anti addition: H and halide add to opposite faces.
The methyl (CH3) group attached to the carbon framework remains fixed as a reference. By locating the chlorine atom’s position relative to the methyl group, one determines whether addition was syn or anti.
Why Are the circled products Enantiomers?
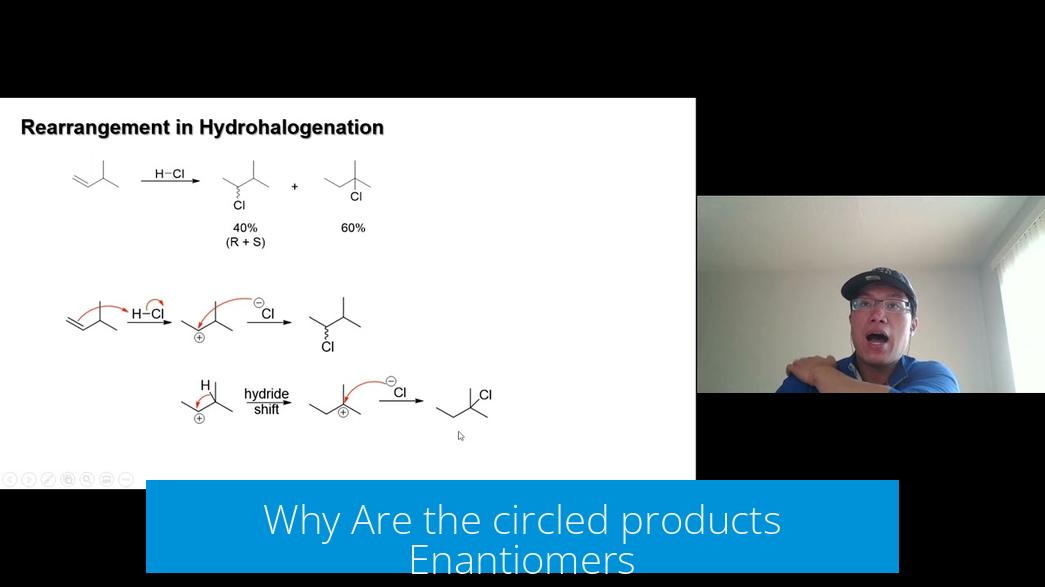
The two circled red structures are nonsuperimposable mirror images. They have the same connectivity but differ in the 3D orientation of atoms, labeling them enantiomers. This occurs because the halogen and hydrogen attach on opposite sides, creating chiral centers.
Where Is the Hydrogen Added?
The halide’s position is visible, but the hydrogen’s location is typically implicit. Since hydrohalogenation adds H and halide across the double bond simultaneously:
- If the halogen is added to one carbon of the double bond, hydrogen adds to the adjacent carbon.
- The hydrogen is positioned on the opposite face of the halogen in anti addition or the same face in syn addition.
Thus, the hydrogen adds across the double bond at the carbon that does not bear the halide. Its stereochemical position complements the halide addition to form either syn or anti stereoisomers.
Summary of Identification Steps
- Fix the methyl group on one side as a stereochemical reference.
- Examine the relative stereochemistry of the halogen substituent.
- Products with halogen and methyl on the same side show syn addition.
- Products with halogen and methyl on opposite sides indicate anti addition.
- Enantiomers arise when these stereochemical arrangements are mirror images.
- The hydrogen adds to the carbon adjacent to the halogen, positioned either on the same or opposite face.
Key Takeaways
- Syn addition products have H and halogen on the same face relative to the methyl group.
- Anti addition products have H and halogen on opposite faces, forming enantiomers.
- Methyl group position serves as a stereochemical anchor to identify addition mode.
- Hydrogen adds to the carbon adjacent to the halogen, opposite or same side depending on addition.
Which product in the hydrohalogenation reaction results from syn addition?
The syn addition product has the chlorine and methyl group on the same side. This means both substituents are added to the same face of the alkene.
How can you identify the product of anti addition in hydrohalogenation?
In the anti addition product, the chlorine and methyl group are on opposite sides. This creates diastereomers with different spatial arrangements.
Why is the methyl group important in distinguishing syn and anti addition products?
The methyl group stays on the same side after alkyl shifts. It serves as a fixed reference to tell whether the chlorine added syn or anti relative to it.
Where is the hydrogen added in the hydrohalogenation reaction?
The hydrogen adds to the carbon opposite the one where chlorine attaches. It is on the opposite side in anti addition or the same side in syn addition.
Are the circled products enantiomers or diastereomers?
The circled products are enantiomers, which are nonsuperimposable mirror images. They differ in spatial arrangement but are related as mirror pairs.




Leave a Comment