Understanding the Difference Between SN1, SN2, E1, and E2 Reactions
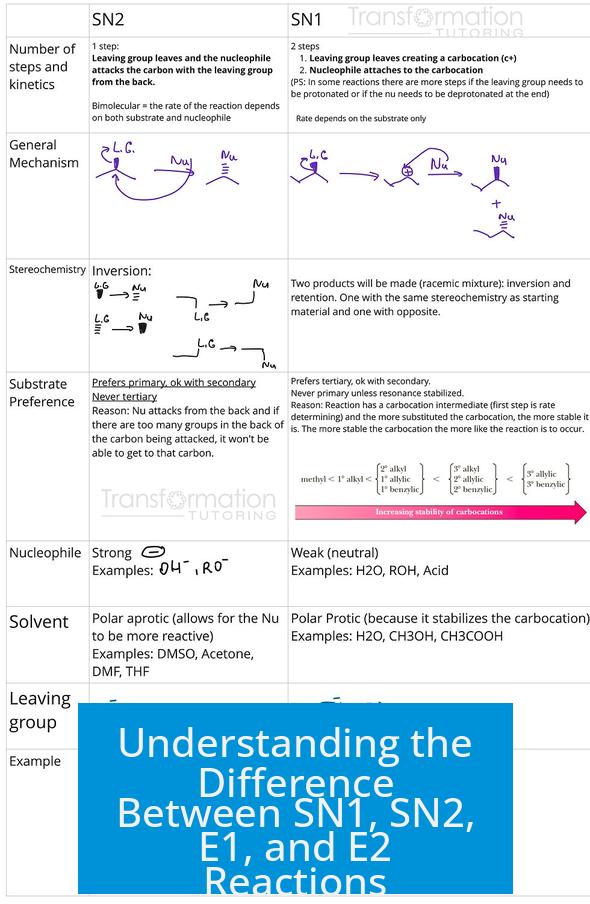
The main difference between SN1, SN2, E1, and E2 reactions lies in their reaction type (substitution vs elimination), molecularity (uni- or bimolecular), mechanism steps (single or multiple), and reagent type (nucleophile vs base). SN1 and SN2 are nucleophilic substitution reactions; E1 and E2 are elimination reactions. SN2 and E2 are bimolecular, single-step mechanisms involving strong nucleophiles or bases, while SN1 and E1 are unimolecular, multi-step mechanisms proceeding through carbocation intermediates and generally involve weak nucleophiles or bases.
1. Types of Reactions: Substitution versus Elimination
- Substitution (SN1 and SN2): A nucleophile replaces a leaving group on a substrate.
- Elimination (E1 and E2): A base removes a proton (deprotonates) adjacent to the leaving group, forming a double bond (alkene).
Both substitution and elimination reactions require the presence of a good leaving group on the substrate.
2. Molecularity and Mechanistic Steps
| Reaction | Mechanistic Steps | Intermediate | Molecularity (Rate-Determining Step) |
|---|---|---|---|
| SN1 | Multistep | Carbocation | Unimolecular (only substrate involved) |
| SN2 | Single step (concerted) | None | Bimolecular (substrate and nucleophile involved) |
| E1 | Multistep | Carbocation | Unimolecular |
| E2 | Single step (concerted) | None | Bimolecular (substrate and base involved) |
For SN1 and E1, the rate-limiting step is the formation of a carbocation after the leaving group departs. This means only the substrate concentration influences the reaction rate. The nucleophile or base attacks or removes a proton after the carbocation forms.
In contrast, SN2 and E2 reactions occur in a single concerted step, meaning the substrate and nucleophile or base act simultaneously. This concerted process produces inversion of stereochemistry in SN2 and requires correct geometry in E2.
3. Role and Strength of Reagents
- SN2 and E2: Strong nucleophiles or strong bases, often negatively charged ions (e.g., OH−, RO−), promote these reactions.
- SN1 and E1: Favor weak nucleophiles or bases (e.g., water, alcohols), often neutral or acidic conditions.
Sometimes reagents can act as either nucleophiles or bases, so identifying the product type helps determine the reaction mechanism. Substitution replaces a group; elimination generates a double bond.
4. Substrate Preferences and Reaction Conditions
- SN2 and E2: Prefer methyl, primary, or secondary alkyl halides due to less steric hindrance facilitating direct backside attacks (SN2) or accessible β-hydrogens (E2).
- SN1 and E1: Favor secondary and tertiary alkyl halides because the carbocation intermediate is more stable in these substrates.
- Temperature Effects: Higher temperatures favor elimination (E1, E2) over substitution.
Heat generally promotes elimination since forming alkenes is entropically favored at higher temperatures.
5. Mnemonics to Recall the Mechanisms
- SN2: Substitution, nucleophilic, bimolecular
- SN1: Substitution, nucleophilic, unimolecular
- E2: Elimination, bimolecular
- E1: Elimination, unimolecular
These mnemonics reflect the nature of the rate-determining step and the type of reaction.
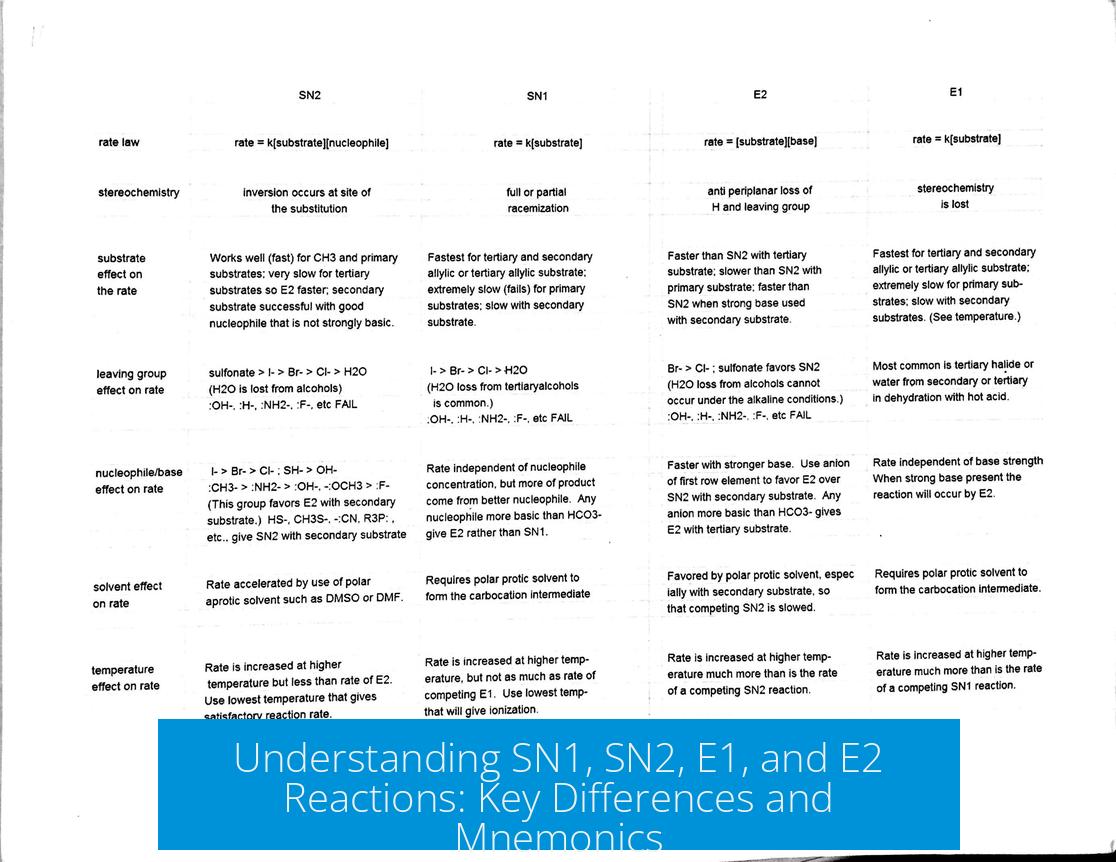
6. Visual Summary
| Property | SN1 | SN2 | E1 | E2 |
|---|---|---|---|---|
| Reaction Type | Substitution | Substitution | Elimination | Elimination |
| Mechanism | Multi-step | Single-step | Multi-step | Single-step |
| Intermediate | Carbocation | None | Carbocation | None |
| Molecularity | Unimolecular (1) | Bimolecular (2) | Unimolecular (1) | Bimolecular (2) |
| Reagent Type | Weak nucleophile | Strong nucleophile | Weak base | Strong base |
| Substrate Preference | Secondary/tertiary | Primary/secondary | Secondary/tertiary | Primary/secondary |
7. Avoid Rote Memorization
Understanding the underlying principles helps link mechanisms to reaction conditions and substrates. Knowing how solvents stabilize carbocations or influence nucleophilicity informs predictions about reaction pathways.
Fundamental concepts such as the stability of intermediates, strength and nature of reagents, steric hindrance, and molecular orbital interactions form the basis for mastering these reactions. Practicing with diverse substrates and conditions consolidates this knowledge.
Key Points to Remember
- SN1 and E1 involve carbocation intermediates and are unimolecular; SN2 and E2 are concerted, bimolecular reactions.
- SN1 and SN2 are substitution; E1 and E2 are elimination reactions.
- Strong nucleophiles/bases favor SN2 and E2; weak nucleophiles/bases favor SN1 and E1.
- Primary substrates favor SN2 and E2; tertiary stabilize carbocations favoring SN1 and E1.
- Product type helps differentiate: substitution replaces groups; elimination forms alkenes.
- Temperature generally encourages elimination reactions over substitution.
- Focus on understanding mechanisms and conditions rather than memorizing reaction types.
What is the main difference between SN1 and SN2 reactions?
SN1 is a multi-step substitution involving a carbocation intermediate and is unimolecular. SN2 is a single-step substitution where the nucleophile attacks as the leaving group leaves, making it bimolecular.
How do E1 and E2 elimination reactions differ in mechanism?
E1 is a two-step process forming a carbocation first (unimolecular), while E2 happens in one step with a base removing a proton as the leaving group leaves (bimolecular).
How do I tell if a reaction is substitution or elimination?
If the product shows a swapped group (nucleophile replaces leaving group), it’s substitution (SN1 or SN2). If a double bond forms by removing a proton and the leaving group, it’s elimination (E1 or E2).
What role do reagent strength and substrate type play in choosing SN1, SN2, E1, or E2?
Strong nucleophiles/bases favor SN2 and E2 on primary or secondary substrates. Weak nucleophiles/bases favor SN1 and E1, often on tertiary or secondary substrates with stable carbocations.
Why is understanding the mechanism better than memorizing differences?
Understanding how nucleophiles, bases, and substrates interact helps predict outcomes, while memorizing alone can cause confusion. Grasping steps, intermediates, and reagent roles is key to mastering these reactions.



Leave a Comment