Understanding the Mechanism of Cyclic Acetal Formation
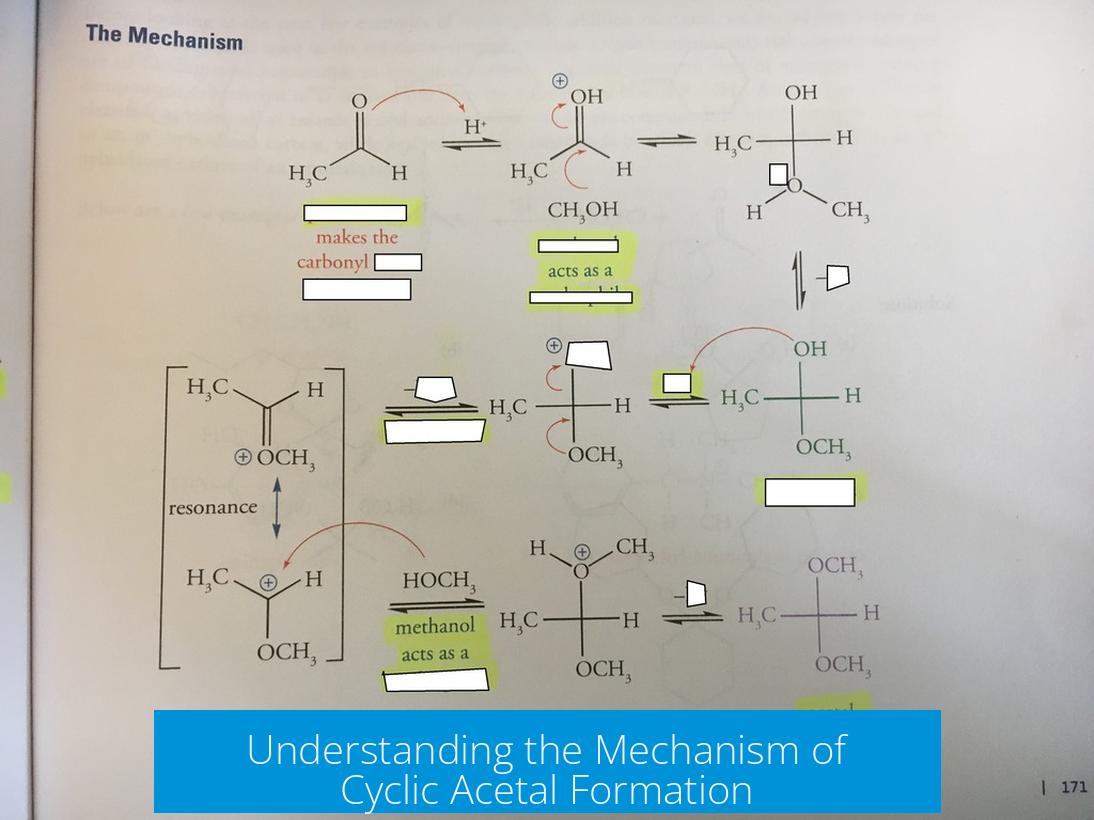
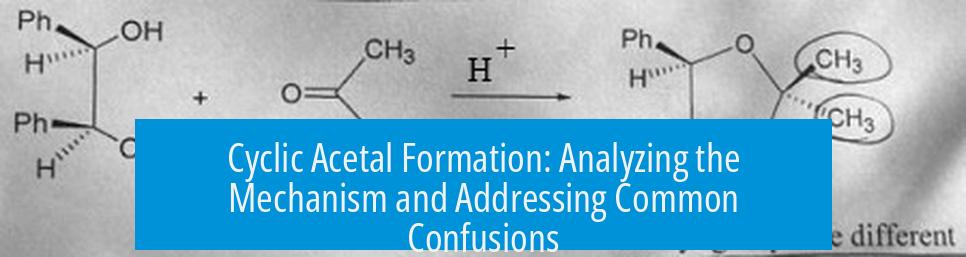
Cyclic acetal formation proceeds through protonation of the carbonyl, nucleophilic attack by a diol, formation of an oxonium intermediate, and intramolecular ring closure, followed by loss of water and eventual ketal formation. This sequence involves a series of proton transfers and bond rearrangements to stabilize intermediates and close the ring structure.
1. Protonation and Water Leaving
The first step is protonation of the carbonyl oxygen, increasing electrophilicity. Water typically does not leave spontaneously; instead, the lone pair on the other hydroxyl oxygen attacks the protonated carbonyl carbon, pushing electrons to eject water and produce an oxonium ion. This step avoids invoking an unstable carbocation intermediate.
2. Intramolecular Ring Closure
The remaining oxygen from the diol attacks the oxonium carbon electrophile intramolecularly. This nucleophilic attack closes the ring, neutralizing the positive charge and forming the cyclic acetal. Resonance contributors of the oxonium intermediate do not alter the fundamental mechanism.
3. Carbocation Concerns and Shifts
Mechanistically, a free carbocation intermediate is unlikely as protonated carbonyls generally undergo direct nucleophilic attack. Formation of such carbocations can provoke rearrangements (like methyl shifts), especially if adjacent to destabilizing groups. Thus, the preferred pathway avoids carbocations by concerted steps.
4. Completion of Ketal Formation
The mechanism often omits a second proton transfer creating a carbonyl intermediate prior to full ketal formation. A proper mechanism includes the reformation of a carbonyl group and a second nucleophilic attack by hydroxyl to stabilize the ketal. This ensures full conversion and ring stability.
5. Summary of Mechanistic Principles
- Water typically leaves after nucleophilic attack rather than spontaneously.
- The oxonium ion is the key electrophilic intermediate stabilized by resonance.
- Intramolecular attack by the diol’s other oxygen closes the ring efficiently.
- Carbocation intermediates are generally avoided due to instability and potential rearrangements.
- complete ketal formation requires further proton transfer and attack steps.
Key Takeaways
- The mechanism should emphasize proton transfers and nucleophilic attacks rather than free carbocations.
- Ring closure occurs through intramolecular nucleophilic attack on a protonated carbonyl forming an oxonium.
- A complete mechanism includes steps for carbonyl regeneration and second nucleophilic attack for ketal stability.
- Electron flow diagrams must show accurate charges and correct electron pushing arrows.
- Choosing one mesomeric form of the intermediate is acceptable; focus on accurate electron flow.
Q1: Should water leave spontaneously in cyclic acetal formation, or is another mechanism preferred?
Water rarely leaves on its own in this reaction. It’s better to show a lone pair on the oxygen pushing electrons to eject water, forming an oxonium ion. This step clarifies correct electron flow and intermediate formation.
Q2: How does the ring form during cyclic acetal formation?
The second oxygen in ethylene glycol attacks the oxonium intermediate. This intramolecular attack forms the cyclic ring and neutralizes the charge, completing the acetal ring structure.
Q3: Is the formation of a carbocation intermediate always necessary in this mechanism?
Direct attack on the protonated carbonyl is more common than carbocation formation. Carbocation intermediates raise concerns about shifts like methyl rearrangements, which are usually not favored here.
Q4: What key steps are often missing or overlooked in practice problems on cyclic acetal formation?
Many miss the second nucleophilic attack needed after reforming the carbonyl to complete the ketal. Including this ensures the mechanism is chemically complete and accurate.
Q5: Does the choice of mesomeric structure for the oxonium ion affect the mechanism?
Both resonance forms of the oxonium intermediate are valid. The important part is consistent electron flow rather than which specific resonance structure is shown.




Leave a Comment