Effective Approach for Determining SN1, SN2, E1, and E2 Mechanisms
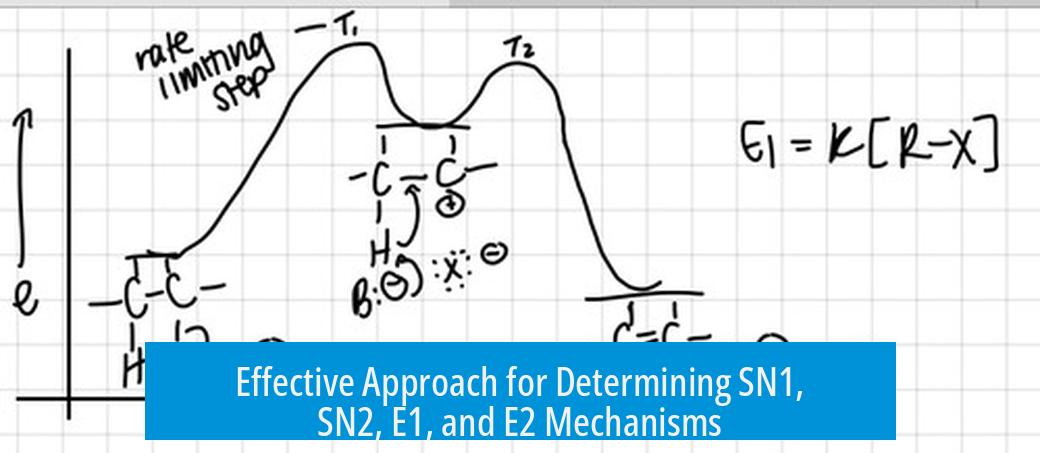
The choice between SN1, SN2, E1, and E2 reaction pathways depends primarily on the substrate structure, nucleophile strength, solvent type, and reaction conditions. This fundamental understanding guides students in predicting the most likely mechanism in organic reactions.
Key Factors Influencing Mechanism Choice
- Substrate Structure: Primary substrates usually favor SN2; tertiary favor SN1 and E1; secondary can undergo any mechanism depending on other factors.
- Nucleophile/Bases: Strong nucleophiles favor substitution (especially SN2), whereas strong bases favor elimination (commonly E2).
- Solvent: Polar protic solvents stabilize carbocations and favor SN1/E1; polar aprotic solvents enhance nucleophile strength, favoring SN2/E2.
Exceptions: Benzyl Bromide and Iodide
Benzyl halides, such as benzyl bromide and iodide, often proceed via SN1 due to resonance stabilization afforded by the aromatic ring. This stabilization promotes carbocation formation despite substrate being potentially primary or secondary, defying the normal SN2 preference.
Carbocation Rearrangements
Carbocation rearrangements, including methyl or hydride shifts, commonly occur when the initially formed carbocation is secondary or less stable. These shifts result in a more stable carbocation intermediate, influencing the final product distribution. Both shifted and non-shifted products may form, but the rearranged product generally predominates.
Secondary Alkyl Halides and Alkoxide Nucleophiles
Secondary alkyl halides present complexity, especially with alkoxide nucleophiles. While polar aprotic solvents usually promote substitution, the strong basicity of alkoxides often favors elimination (E2) pathways. This nuance explains variability in teaching approaches and highlights the importance of base strength in these systems.
Summary of Key Points
- Reaction mechanism depends on substrate, nucleophile/base strength, and solvent.
- Benzyl halides undergo SN1 due to resonance stabilization of carbocations.
- Carbocation rearrangements occur to form more stable intermediates, affecting product ratios.
- Secondary alkyl halides with strong bases like alkoxides favor elimination (E2) even in polar aprotic solvents.




Leave a Comment