Is My Understanding of the Difference Between a Reaction and a Mechanism Correct?

Your understanding is essentially correct. A reaction describes the overall process of converting reactants into products under specific conditions. A mechanism explains how this conversion happens step-by-step at the molecular level.
What is a Reaction?
A reaction defines the transformation of one or more substances into different substances. It includes the reactants, products, and the conditions needed to carry out the change. For example, when methanol (CH3OH) reacts with hydrobromic acid (HBr) and heat, it forms methyl bromide (CH3Br) and water (H2O). The reaction specifies:
- The starting materials (reagents)
- The products formed
- Conditions such as temperature, solvent, and time
An esterification reaction illustrates this well. A carboxylic acid reacts with an alcohol in the presence of an acid catalyst to produce an ester and water.
What is a Mechanism?
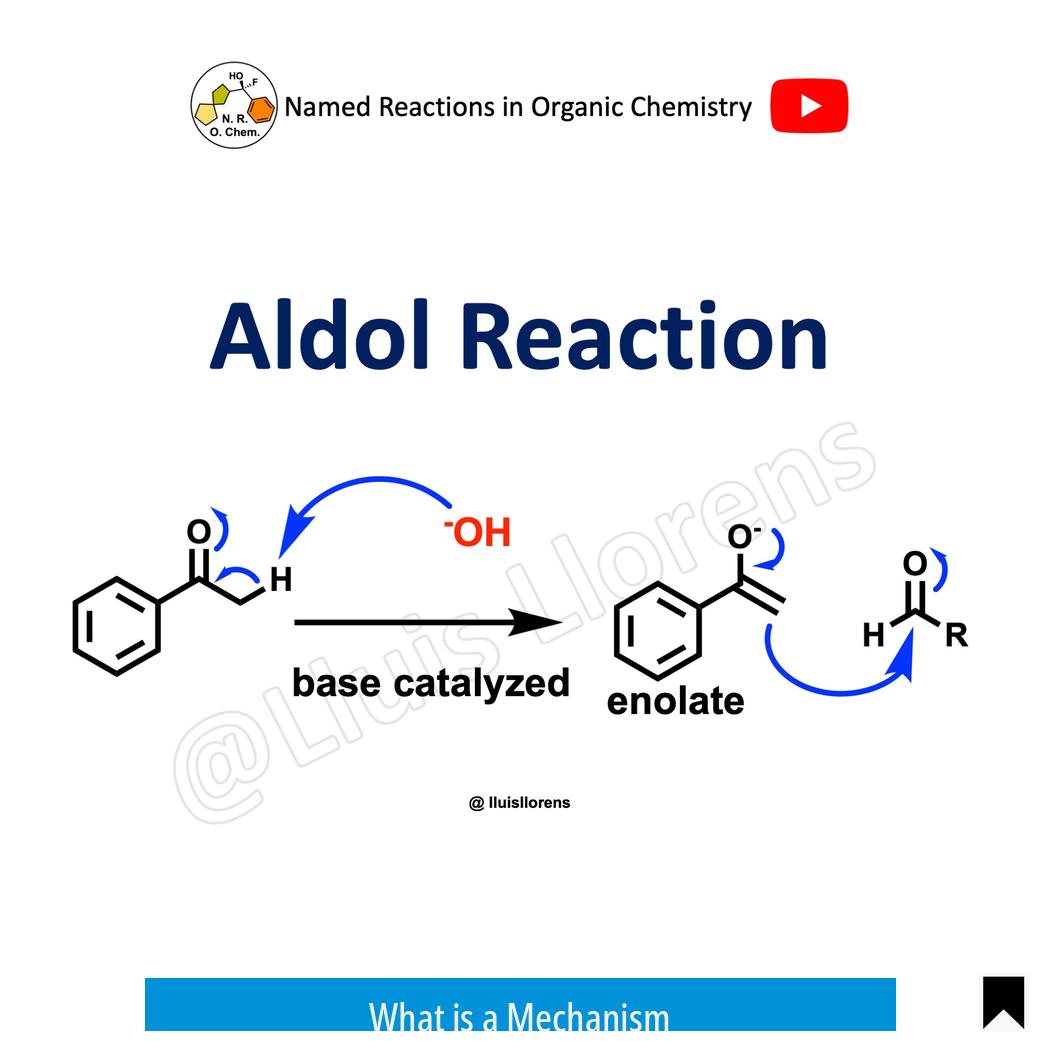
A mechanism describes the detailed sequence of bond-breaking and bond-forming events that convert reactants into products. It shows the intermediate structures and electron movements, often represented by curved arrows. Mechanisms are based on experimental evidence and help explain the reaction pathway.
For example, the mechanism of esterification involves multiple steps:
- Protonation of the carboxylic acid
- Nucleophilic attack by the alcohol
- Proton transfers
- Elimination of water
This stepwise pathway explains how the ester forms at the molecular level.
How Are Reactions, Mechanisms, and Synthesis Related?
Synthesis involves multiple reactions connected in a sequence to build a target molecule. Each reaction proceeds via one or more mechanisms that detail the underlying steps. Understanding both reactions and mechanisms is crucial for designing and controlling synthetic pathways.
Key Takeaways
- A reaction describes the overall chemical transformation, listing reactants, products, and conditions.
- A mechanism details the stepwise molecular events showing how bonds break and form.
- Mechanisms rely on experimental data and use curved arrows to illustrate electron movements.
- Synthesis combines multiple reactions, each occurring through specific mechanisms.
Is My Understanding of the Difference Between a Reaction and a Mechanism Correct?
Short answer? Yes, your grasp of the difference between a reaction and a mechanism is spot on. A reaction outlines what changes, while a mechanism reveals how those changes happen — the behind-the-scenes choreography of molecules. Let’s unpack this in detail, add depth, and clear any fog.
Imagine you’re cooking. The recipe is like a chemical reaction — it tells you the ingredients (reactants), the final dish (products), and some cooking conditions (temperature, time, solvent). The step-by-step cooking method is akin to the mechanism — the detailed process explaining how ingredients combine, transform, and turn into the final meal.
What Exactly Is a Reaction?
A reaction is the overall change happening between starting materials and end products. Simply put, it answers: “What goes in?” and “What comes out?” but it doesn’t dive into the molecular drama that unfolds between those points.
For instance, when methanol (CH3OH) reacts with hydrobromic acid (HBr) under heat, it produces methyl bromide (CH3Br) and water (H2O). The reaction can be summarized as:
CH3OH + HBr + heat → CH3Br + H2O
Notice how this equation tells you the players and conditions, but not exactly how those atoms rearranged to form new compounds. That’s the magic the mechanism uncovers.
Another classic example is esterification, where a carboxylic acid reacts with an alcohol in the presence of an acid catalyst. The result? An ester and water. This is the reaction:
Carboxylic acid + Alcohol + Acid catalyst → Ester + Water
Again, this overview doesn’t tell you about the microscopic moves inside molecules. We just know the start, the end, and some reaction conditions like solvent, temperature, and time.
Delving Into the Mechanism: The Molecular Playbook
A mechanism, on the other hand, is a detailed map of the journey from reactants to products. It shows every bond that breaks and every bond that forms during the transformation. In organic chemistry, mechanisms often involve curved arrows illustrating electron flow — essentially storyboards of chemical changes.
Take the esterification reaction we just mentioned. The mechanism would show these key steps:
- Protonation of the carboxylic acid – adding a proton to increase electrophilicity.
- Nucleophilic attack by the alcohol – oxygen from alcohol attacks the protonated acid.
- Proton transfers – rearranging protons to stabilize intermediates.
- Elimination of water – removing a water molecule to form the ester bond.
This sequence tells you more than what changed—it reveals how it changed, step by step. Arrows tracing electrons show the movement causing bond formation and cleavage.
Trying to swallow this whole mechanism at once might feel like following a plot twist in a mystery novel, but it’s fascinating. Every arrow explains the logic behind molecular behavior, supported by experimental data. So yes, mechanisms are based on real evidence, not just guesses.
How Do Reactions, Mechanisms, and Synthesis Interrelate?
In organic chemistry, synthesis is like building a complex puzzle. You string multiple reactions together, each with its own mechanism. A synthetic pathway can involve dozens of reactions — every step a mini transformation with its own molecular storytelling.
To put it simply:
- Reactions tell you the big picture — reactants, products, and conditions.
- Mechanisms give the microscopic details — the molecular ballet performing behind the scene.
- Synthesis is the series of those reactions, piecing them together to create complex molecules.
Without understanding mechanisms, synthesis is like throwing ingredients into a pot and hoping for edible results. With mechanisms, chemists can design more efficient, selective, and controlled synthesis routes — by predicting which bonds will form or break at each stage.
Why Does This Matter? Practical Tips and Insights
You might wonder, “Why not just memorize reactions and call it a day?” Knowing the mechanism has several perks:
- Predicting side products: Some reactions produce unwanted byproducts. Mechanisms explain how and why, helping you tweak conditions to minimize waste.
- Designing new reactions: Understanding molecular steps allows chemists to innovate new synthetic routes instead of reinventing the wheel.
- Problem-solving: Stuck reaction? Sometimes mechanisms point to why a reaction stalls or reverses.
From personal experience, students grasp mechanisms better when relating them to visible “electron flow” using curved arrows. It turns abstract chemistry into a detective story unraveling molecular secrets.
Wrapping Up: Your Understandings Are Correct — And Here’s the Takeaway
Your basic idea — that the reaction is the ‘what and when’ while the mechanism is the ‘how and why’ — captures the essence perfectly. Reactions summarize the start and finish under given conditions. Mechanisms narrate each microscopic move leading to that finish. Syntheses string multiple such reactions and mechanisms together.
Next time you see a reaction equation, imagine the bustling molecular steps it hides. And when studying synthesis, think of it as storytelling interwoven with chemical precision.
Ready to dive deeper? Grab some reaction mechanisms, follow the arrows, and become a molecular storyteller yourself!





Leave a Comment