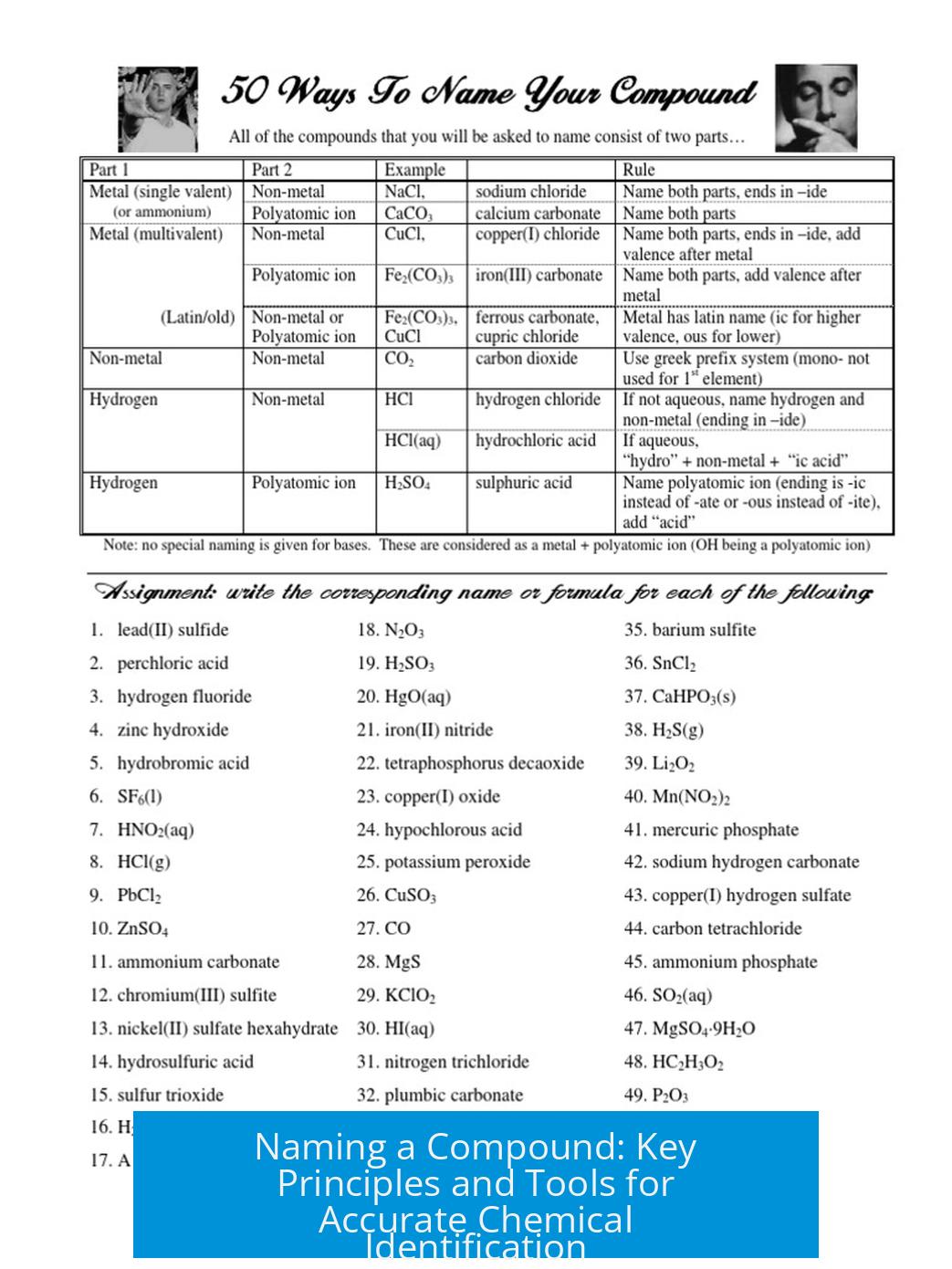
Naming a Compound: Key Principles and Tools
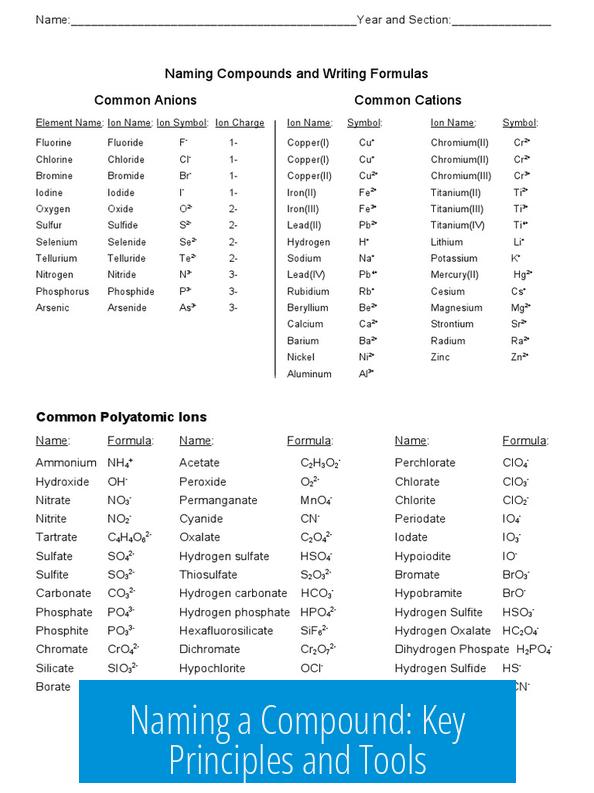
Naming a compound requires an accurate molecular structure that respects carbon’s bonding limits and geometry. Carbon atoms form only four bonds. Structures showing pentavalent carbons—carbons with five bonds—do not exist in reality. Such invalid structures cannot be named correctly in chemistry.
Carbon Bonding Rules and Molecular Validity
Carbon’s tetravalency is essential. Each carbon atom can form up to four single bonds, double bonds counting as two bonds, or triple bonds as three. Any structure with carbons having more than four total bonds contradicts basic chemistry principles.
For example, a compound depicting three pentavalent carbons is impossible. This affects naming because the International Union of Pure and Applied Chemistry (IUPAC) only names chemically plausible compounds. Geometry also influences stability and existence; correct 3D arrangements are necessary for valid molecules.
Accounting for Structural Features in Naming

Naming must incorporate all structural features, including double bonds. For instance, carbon-carbon double bonds influence the compound’s suffix and numbering. Ignoring these will result in incorrect names that misrepresent the molecule’s true structure.
Using Digital Tools for Accurate Naming
- Several online platforms help generate valid compound names from drawn molecular structures.
- The ChemSpider Structure Search is a useful tool where users can draw compounds and receive proper IUPAC names.
- These tools are invaluable for organic chemistry students and professionals for verifying structural validity and naming accuracy.
Examples of Valid Structural Cores

When naming cyclic compounds with double bonds, accurate cores must be identified. For example, cyclopentadiene is a five-membered ring with two double bonds. It serves as a realistic scaffold for many derivatives and must be considered when naming related compounds.
Variations around cyclopentadiene respect bonding rules by maintaining the correct number of single and double bonds and never placing double bonds adjacently without proper considerations.
Summary of Key Points
- Carbon forms only four bonds; pentavalent carbons are invalid.
- Compound names must reflect double bonds and structural features.
- Geometry impacts molecular existence and naming correctness.
- Online tools aid in drawing and naming accurate compounds.
- Cyclopentadiene represents a common valid core for naming related cyclic compounds.
Naming a Compound: The Carbon Conundrum & How to Get It Right

Naming a compound—it sounds straightforward until you draw a molecule with three carbons, each boasting five bonds. Wait, what? That’s impossible! Carbon atoms simply aren’t built for pentavalency. They max out at four bonds. So, before naming your masterpiece, you must consider whether the molecule can even exist.
Let’s dive into this fascinating subject and explore how carbon bonding rules, molecular geometry, and double bonds influence how a compound is named. Plus, discover clever tools and real-life examples that make your compound naming journey smoother.
Carbon’s Four-Bond Limit: The Non-Negotiable Rule
Here’s a fun fact: carbon laughs in the face of pentavalency. It only forms four bonds, period. If you sketch a molecule showing five bonds on a single carbon, it’s like trying to make a cat bark. It just can’t happen. For example, if your molecular structure has three carbons with five bonds each, that molecule is a no-go.
This is crucial when naming compounds because you have to start with a valid structure. The four-bond rule is fundamental. Alongside bonding, the geometry—how atoms connect in 3D space—also affects the molecule’s viability. Even if your bond count looks right, if the geometry clashes, your molecule remains fictional.
Ever wondered why some compounds exist only as theory? Often, they break these bonding or geometry rules.
The Double Bond Dilemma: Don’t Ignore Them in Names
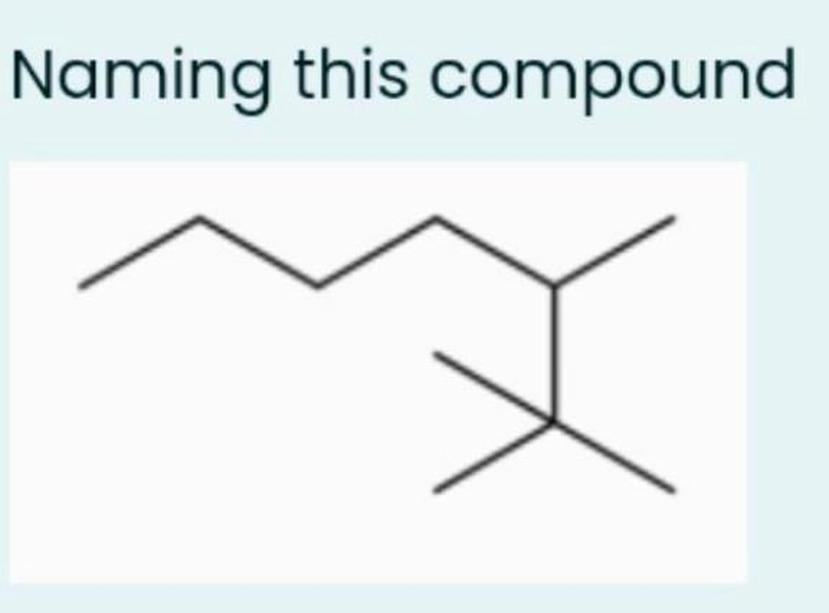
Double bonds aren’t just fancy decorations on your molecule; they influence the name heavily. Your compound’s name must reflect them accurately. If you ignore double bonds between carbons, you’re shortchanging the molecule’s identity.
For instance, take cyclopentadiene—the superstar of five-membered rings with two double bonds. If you fail to include the “diene” in the name, you miss out on the crucial chemical character of the molecule. Naming is about capturing every nuance, and double bonds are key players.
Need Help? Online Tools Can Save Your Sanity
Let’s be honest: organic chemistry nomenclature can cause headaches. Luckily, there’s a lifesaver. ChemSpider’s structure search tool lets you draw your compound and spits out the correct IUPAC name. It’s like having a naming expert on speed dial.
Why guess and stress when a reliable tool can verify your structure’s validity and generate a proper name? This tool doesn’t just save time; it helps you avoid embarrassing mistakes, like naming impossible compounds.
Picking a Realistic Core: Cyclopentadiene and Its Variants
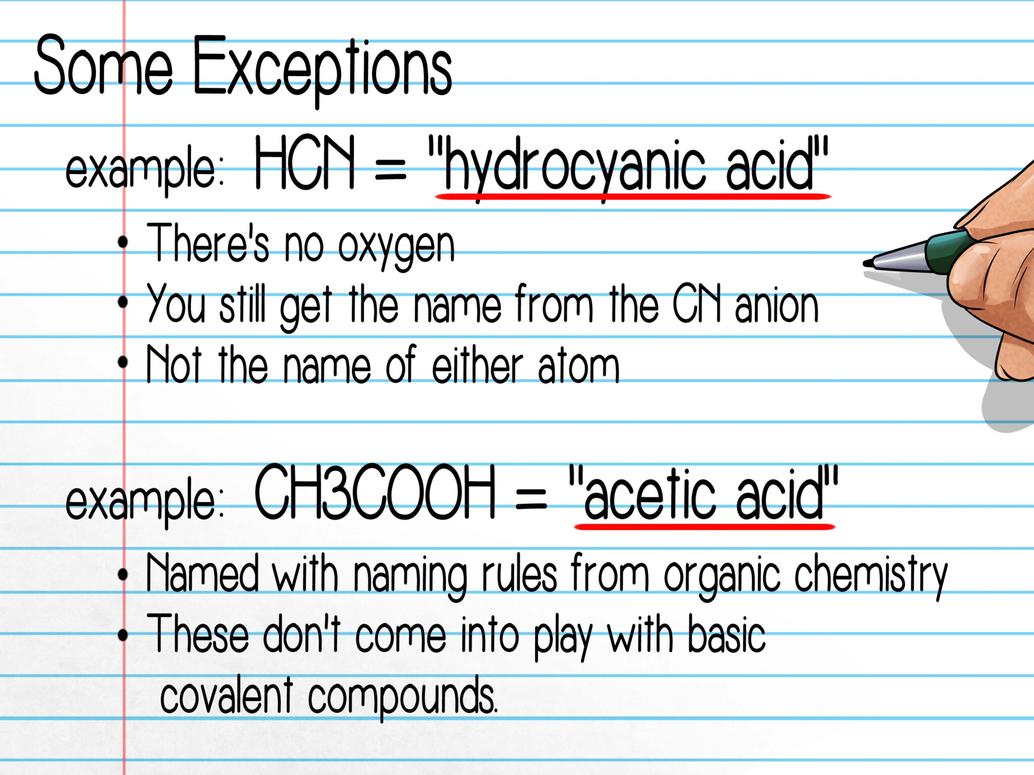
If you’re stuck with an impossible structure, look for a close real-world cousin. Cyclopentadiene is a fantastic starting point. It’s a five-carbon ring with two double bonds, neatly fitting carbon’s bonding rules.
According to Sigma-Aldrich’s resource, cyclopentadiene exhibits lots of variations. But here’s a golden rule: substituents attach to single bonds, never to adjacent double bonds.
This insight is crucial. It reminds you to respect molecular stability in naming and structure. If your sketch tries to force substituents onto neighboring double bonds or create pentavalent carbons, adjust it. Aim for that stable core first, then build thoughtfully.
The Intersection of Geometry and Bonding: More Than Just Counting Bonds
Just having four bonds per carbon isn’t enough—geometry plays a starring role. Imagine trying to create a shape where the angles don’t fit carbon’s tetrahedral or planar preferences. The molecule won’t stand a chance.
So, when learning to name compounds, develop an intuition for not just bond counts but spatial arrangement. The molecule’s name often encodes these geometric realities through prefixes and suffixes indicating double bonds, ring structures, or substituents.
Practical Tips to Nail Compound Naming
- Start by checking the carbon bonding: no more than four bonds per carbon.
- Identify all double bonds and rings; include them explicitly in the name.
- Use online tools like ChemSpider’s structure search to validate your molecule and get the official name.
- Compare your structure to known compounds like cyclopentadiene for a realistic foundation.
- Remember, substituents attach to single bonds and avoid placing them next to each other on double bonds.
Why Does This Matter? The Story Behind the Name
Imagine you’re a chemist working to synthesize a new drug. You send your proposed structure to a colleague, and they spot pentavalent carbons. They chuckle and ask if you made a typo. Naming compounds correctly isn’t just academic snobbery; it affects communication, research replication, and safety.
An incorrect name can send you on wild chemical goose chases or worse, cause you to synthesize something unstable or non-existent. Precision in naming anchors chemistry in reality.
Final Thoughts: Naming Compounds Is About Respecting Chemistry’s Rules
Effective compound naming starts with respect for carbon’s four-bond limit and molecular geometry. Overlooking these basics creates impossible molecules and meaningless names. Double bonds must be factored in, substituents carefully placed, and online tools wisely used.
Next time you sketch a compound, ask yourself: “Does this molecule respect carbon’s bonding rules? Are the double bonds accounted for? Is the core structure realistic—maybe a cyclopentadiene derivative?” Answer these, and naming your compound becomes a much less daunting task.
So, embrace the rules, use available tools, and enjoy watching chemistry unfold through proper naming. Who knew naming a compound could be this intriguing?
What is the significance of carbon’s bonding capacity in naming compounds?
Carbon can only form four bonds. If a structure shows carbon with five bonds, it is invalid. Naming such impossible structures leads to errors because they don’t exist in reality.
How do double bonds affect the naming of a compound?
Double bonds must be included in the compound’s name. Ignoring them creates an incorrect or incomplete name because the presence of double bonds changes the molecule’s structure and properties.
Can online tools help name chemical compounds correctly?
Yes. There are websites where you can draw the compound, and they will provide its correct name. These tools are useful for verifying structures and ensuring accurate names in organic chemistry.
Why can’t the compound with three pentavalent carbons exist?
Because carbon atoms can only have up to four bonds, having three carbons with five bonds each violates bonding rules. This makes the compound impossible and unnameable in standard chemistry.
What is a realistic example related to naming cyclopentadiene derivatives?
The closest real structure is cyclopentadiene. Variations exist, but bonds next to double bonds are always single. This ensures the molecule follows bonding and geometric rules.




Leave a Comment