Understanding Resonance Structures: Why Does the Negative Charge Move?

Resonance describes the movement of electrons within a molecule, not the literal shifting of positive or negative charges themselves. If your example shows a negative charge moving through resonance forms, it is because the structure represents an anion system, where electrons are delocalized rather than just the charge itself migrating.
Resonance and Charge Types: Differentiating Cations and Anions
Resonance structures can involve positive charges (cations), negative charges (anions), or neutral molecules. The key is to recognize what type of species the resonance applies to. Often, chemistry students first encounter resonance with positively charged species where the positive charge appears to move through the structure.
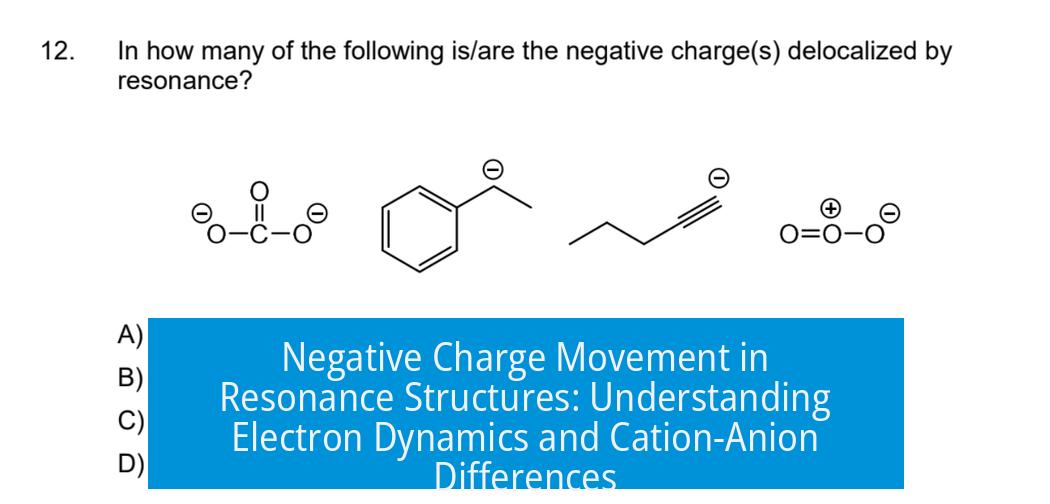
In contrast, if your molecule is an anion (has a negative charge), the resonance structures depict the movement of excess electrons, not a moved positive charge. For example, resonance in carboxylate ions shows the negative charge delocalized between two oxygen atoms. This contrasts with resonance in carbocations where a positive center shifts.
How Electron Movement Drives Resonance, Not the Physical Movement of Charges
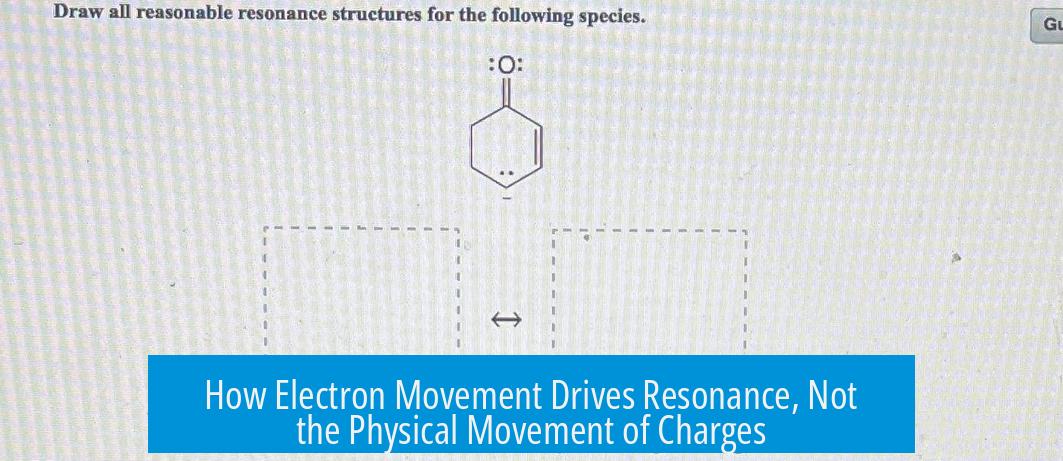
- Resonance involves shifting electrons, usually through pi bonds or lone pairs.
- Formal charges in resonance structures do not physically move but result from electron rearrangements.
- A charge appearing to “move” is actually the consequence of bond making and breaking, altering where the extra or deficient electrons reside.
In essence, resonance structures are different depictions of the same molecule’s electron distribution. They illustrate how electrons spread to stabilize the system by occupying different regions.
Why is the Negative Charge Moving in This Example?
When the example involves an anion, the lone pair on the negatively charged atom (often oxygen or carbon) participates in resonance. This lone pair electrons can form a new pi bond with an adjacent atom, pushing the bonding electrons elsewhere. The electrons cascade through the conjugated system as follows:
- A lone pair on oxygen forms a double bond with a carbon.
- The existing double bond between two carbons breaks, pushing its electrons onto an adjacent carbon as a lone pair.
- This results in a new location of negative charge, as the carbon with the new lone pair carries the extra electron.
- The process repeats along the conjugated chain, delocalizing the negative charge through resonance.
This movement is governed by the availability of electrons in lone pairs and pi bonds within the conjugated system. The negative charge appears to move because the extra electrons occupy different atoms as bonds rearrange.
Conjugation and Resonance Stabilization
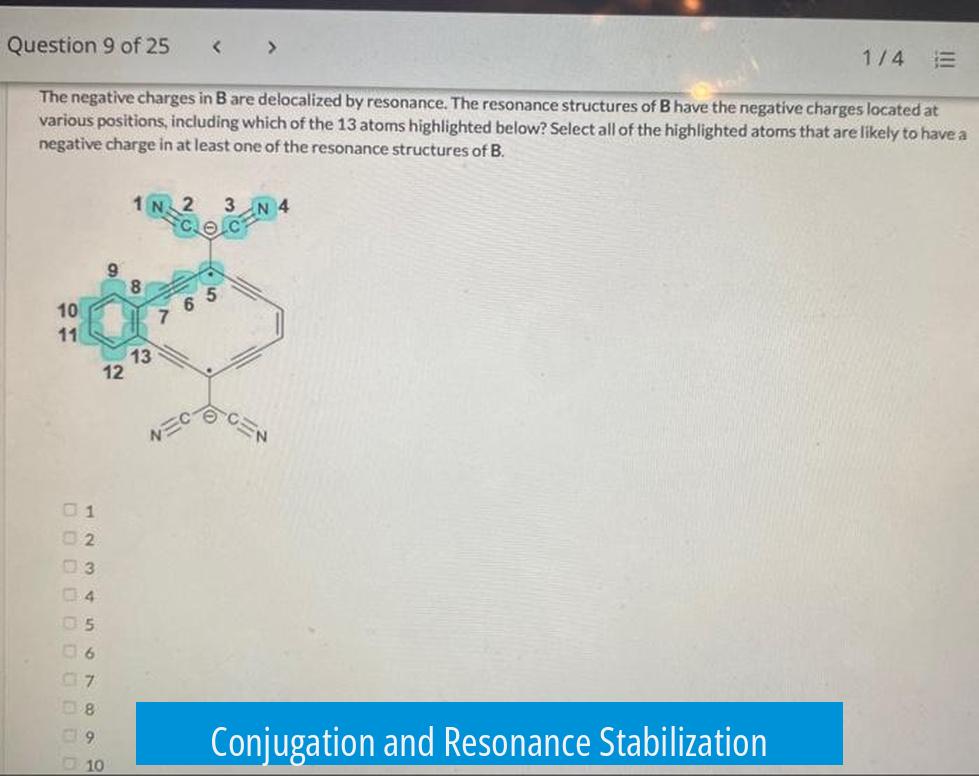
Resonance only happens in conjugated systems where pi bonds or lone pairs can overlap across adjacent atoms. This conjugation allows electrons to delocalize, which stabilizes the molecule.
In molecules like carboxylate ions or enolate ions, resonance distributes the negative charge over multiple atoms. This distribution lowers the energy of the molecule and increases stability.
Similarly, benzene, a neutral molecule, experiences resonance by sharing electrons equally across its six carbon atoms. This equal sharing contributes to its remarkable stability.
Summary Table: Charge Type and Resonance Electron Movement
| Charge Type | Electron Movement | Effect on Resonance Structures |
|---|---|---|
| Positive Charge (Cation) | Electrons shift away from positive center | Positive charge appears to move as bonds form and break |
| Negative Charge (Anion) | Lone pair electrons form new bonds; pi bonds shift | Negative charge appears on different atoms via electron delocalization |
| Neutral Molecule | Electron pairs in pi bonds delocalize | Stabilizes structure without formal charge movement |
Practical Insights for Learning Resonance
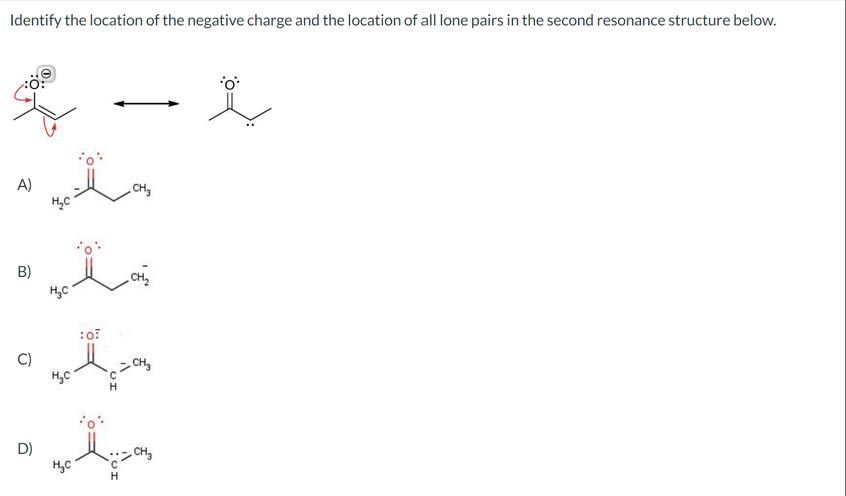
Understanding resonance deeply benefits from knowledge of Molecular Orbital (MO) theory. This explains electron delocalization as the formation of orbitals spread over several atoms.
Practice by drawing structures with lone pairs and pushing electrons logically. Use electron-pushing arrows to track how electrons form or break bonds, not how charges physically move. Recognizing charge movement as a result of electron flow clarifies many resonance problems.
Seek guidance from instructors, especially in office hours, to solidify these principles when confusion arises.
Key Takeaways
- Resonance involves electron movement, not physical shifting of charges.
- Negative charge moves in resonance structures by electron pairs relocating in conjugated systems.
- The difference between anion and cation resonance lies in which electrons shift and where formal charges appear.
- Drawing resonance involves shifting lone pairs and pi bond electrons to generate valid resonance forms.
- Resonance stabilizes molecules by delocalizing electrons, distributing charge across atoms.
Resonance Structure: Why Is the Negative Charge Moving, Not the Positive?

So you thought positive charges are the ones that dance through resonance structures, right? Well, in this case, it’s the negative charge showing off its moves. Confused? You’re not alone. Most chemistry learners first meet resonance through cations—molecules carrying positive charges—so naturally, they associate charge movement with positive charges hopping around. But resonance, like a good party, has different guests. Sometimes the negative charges get to boogie.
Let’s pull back the curtain on this electron choreography.
Resonance Is All About Electrons, Not Charges
First, a crucial point clears the fog: resonance doesn’t involve charges physically moving from atom to atom. Instead, it’s the electrons—the little builders of chemical bonds—that shuttle around. This electron shuffle changes the bonding patterns, which then alters where formal charges appear.
Consider resonance as a quick shuffle of electrons without atoms themselves moving. This shuffle can make a previously neutral atom look negative or positive on paper depending on electron placement, but the actual charge doesn’t float through the molecule like a charged ping-pong ball.
So, why do you see negative charge moving here? Because the electrons responsible for that negative charge are the ones hopping about!
The Difference Between Cation and Anion Resonance
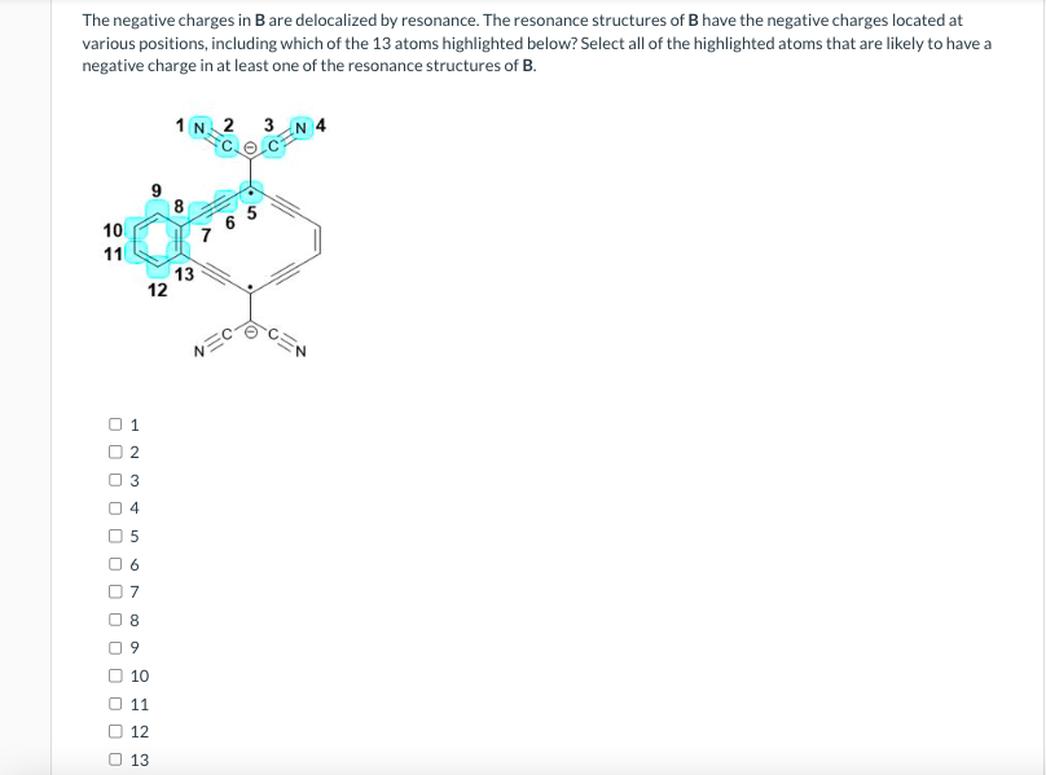
When your textbook or professor shows resonance with positively charged molecules (cations), the positive charge seems to drift across the structure. That’s simply because the resonance forms of that molecule distribute the electron deficiency across different atoms. But here’s the kicker: in your current example, the molecule carries a negative charge. It’s an anion.
Imagine you’re watching a dance where the star dancers used to be “positive” but now, the spotlight shifts to “negative” dancers. The rules change a bit because the negative charge arises from extra electrons—those who literally crash this electron party and make the molecule negatively charged.
Hence, you naturally see the negative charge moving, as the lone pairs and π electrons redistribute along the molecule’s conjugated system.
Why Does Negative Charge Move in This Specific Case?
Now, to the nitty-gritty reason—the negative charge seems to move because the electrons are moving through conjugated bonds.
Picture this: you have oxygen atoms bearing lone pairs beside double bonds with carbons. Those lone pairs on oxygen don’t just sit idly—they push electrons down to form double bonds with adjacent carbons. This movement of electrons breaks other bonds elsewhere, shifting electrons onto new atoms, creating a chain of electron shifts.
Each step in this dance drives the negative charge along the carbon chain because when electrons land, the atom gains a negative formal charge (an extra electron), and where electrons leave, the charge shifts accordingly.
- Step 1: On oxygen, a lone pair forms a new double bond.
- Step 2: The adjacent carbon-carbon double bond breaks, and the electrons slide onto another carbon.
- Step 3: This process continues, pushing the negative character along the chain.
This is not random; it follows the molecule’s conjugated system. Each atom negative in the resonance structure holds onto a lone pair, making them richer in electrons than usual.
So, in this resonance hybrid of an anion, the extra electrons travel through the π bonds. You don’t see a positive charge because this molecule isn’t a cation. It’s an anion, so the electron “moves” paint the negative charges’ path.
Conjugation and Resonance: Electron Stability by Sharing
Resonance often shows conjugated systems—atoms connected by alternating single and double bonds allowing electrons to roam across those π bonds. This roaming electron cloud stabilizes molecules.
Why’s that important? Because dispersing electrons is like sharing a large pizza instead of each atom getting its mini pie—everyone ends up happier (more stable) with the shared electrons.
For example, benzene, a classic resonance hero, is a neutral molecule with electrons delocalized evenly, giving it extraordinary stability. Similarly, in your example, negative charge shifts through conjugated bonds, helping the molecule stabilize extra electrons.
Practical Tip: Get Hands-On and Draw Resonance!
If this still sounds like a strange electron dance troupe, grab a pencil and paper. Mark lone pairs on oxygen, and use curved arrows to ‘push’ electron pairs:
- Push electrons from lone pairs or double bonds toward adjacent atoms.
- Watch which bonds break and which electrons end up where.
- Track the formal charges on atoms after each electron shift.
Visualizing these electron moves will clear up the “Why is the negative moving instead of the positive?” question and reinforce that it’s always about electrons sliding around, not charges hopping like traffic cops on duty.
A Quick Note for Learners: MO Theory Lightens the Path
If resonance makes your head spin, you’re not alone. A little dose of molecular orbital (MO) theory might help. MO theory explains atomic orbitals merging to form bonding and antibonding molecular orbitals. This framework makes electron delocalization and resonance more intuitive.
Your best bet? Don’t hesitate to schedule office hours with your professor to go over resonance examples in depth. They can offer guidance tailored to your learning style and clarify confusing points live. After all, seeing these concepts in action beats memorizing abstract rules.
Summing It Up: Negative vs. Positive Charge Movement in Resonance
Resonance structures don’t show charges physically moving—rather, the movement of electrons changes formal charges along the molecule. Whether it’s a positive charge or a negative charge resonates is determined by the molecule’s identity: cations versus anions. In your case, the negative charge drifts because you’re dealing with an anion rich in electrons that hop through conjugated π bonds.
Thinking of resonance as electron movement rather than charge movement clears up many common misunderstandings. It’s electrons that form and break bonds in constant rearrangement, creating resonance hybrids that help explain molecular stability and reactivity.
Next time you encounter resonance, ask yourself: “Where are the electrons going, and how does that shift formal charges?” That approach keeps you on solid footing—no matter what charge shows up in your molecule’s dance.
So, what’s your next resonance challenge? Share it, and we’ll decode those electrons’ secret moves!
Why is the negative charge moving instead of a positive charge in this resonance structure?
This example involves an anion, not a cation. The negative charge moves because electrons shift through the molecule. Resonance moves electrons, not the charges themselves.
Does resonance always involve charges moving around the molecule?
Resonance is about electrons moving to form different bonds. The formal charge may change location, but it’s the electrons that shift. Charges appear to move but really it’s the bonding electrons rearranging.
Can resonance involve both positive and negative charges or even neutral molecules?
Yes. Resonance can stabilize systems with positive charges, negative charges, or no charges at all. The key is electron delocalization in conjugated systems.
How do lone pairs contribute to the movement of negative charge in resonance?
Lone pairs on atoms, like oxygen, can be pushed to form double bonds. This shifts electrons and moves the negative charge through the structure, creating resonance forms with different electron placements.
What role does conjugation play in the movement of negative charge during resonance?
Conjugation allows electrons to spread over multiple atoms, stabilizing the negative charge. As electrons shift along double bonds and lone pairs, the negative charge appears to move through the conjugated system.



Leave a Comment