Does Resorcinol Experience a Positive Mesomeric Effect?
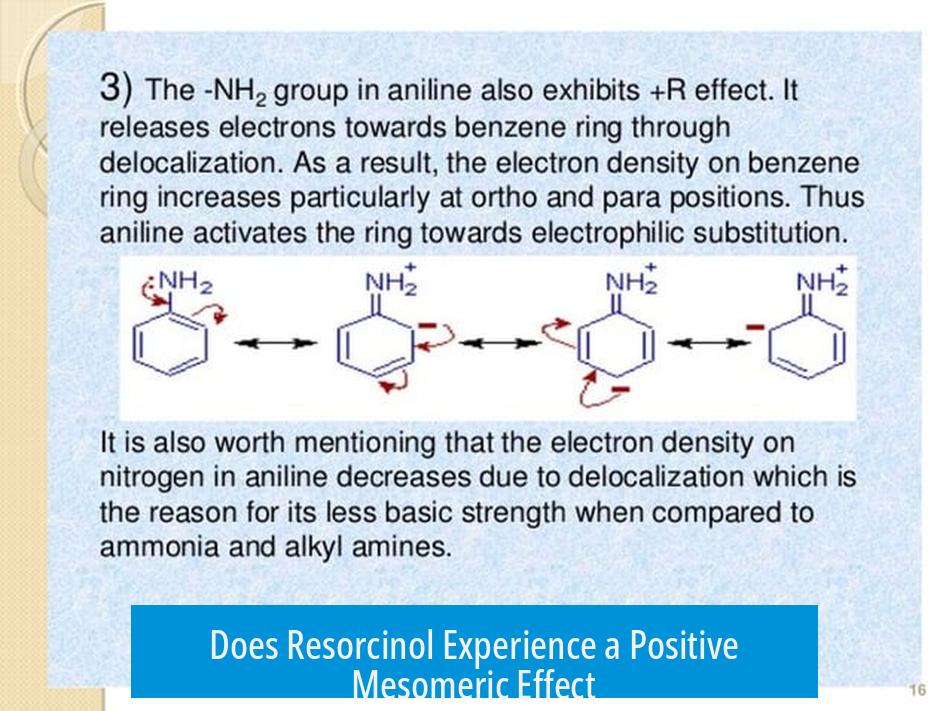
Resorcinol does not experience a positive mesomeric effect from its meta hydroxyl group. This conclusion arises from the electron donating nature of hydroxyl groups and the resonance behavior within the benzene ring system. Unlike ortho and para positions, the meta position limits resonance stabilization by the hydroxyl substituent. Analysis of resonance structures, experimental acidity data, and electron density distribution confirms this.
Understanding Mesomeric (Resonance) Effects
Mesomeric effects describe how substituents influence electron density in aromatic rings through resonance. Electron donating groups (+M effect) push electron density into the ring, stabilizing intermediates and altering properties like acidity and reactivity. Electron withdrawing groups (-M effect) pull electron density away. The hydroxyl (–OH) group typically donates electrons via resonance.
Hydroxyl Group as an Electron Donor
- The hydroxyl group donates electrons through its lone pairs on oxygen.
- This resonance donation usually increases electron density at ortho and para positions.
- Electron donation often reduces acidity by stabilizing positive charges in intermediates.
However, the position of the substituent relative to the reactive site is critical. Resonance effects appear strongly at ortho and para carbons but are negligible or different at meta positions.
Position-Dependence of Resonance in Resorcinol
Resorcinol (1,3-dihydroxybenzene) has hydroxyl groups at the 1 and 3 positions, meaning one hydroxyl occupies a meta position relative to the other.
Studies show:
- The ortho and para carbons benefit from resonance electron donation by the hydroxyl.
- Meta carbons experience mainly inductive effects, namely slight electron withdrawal.
- The meta hydroxyl group in resorcinol does not contribute to resonance electron donation at meta carbons.
This means the meta hydroxyl acts differently than the ortho or para hydroxyls with respect to resonance interaction.
Resonance Structures and Electron Density: Phenolates as Models
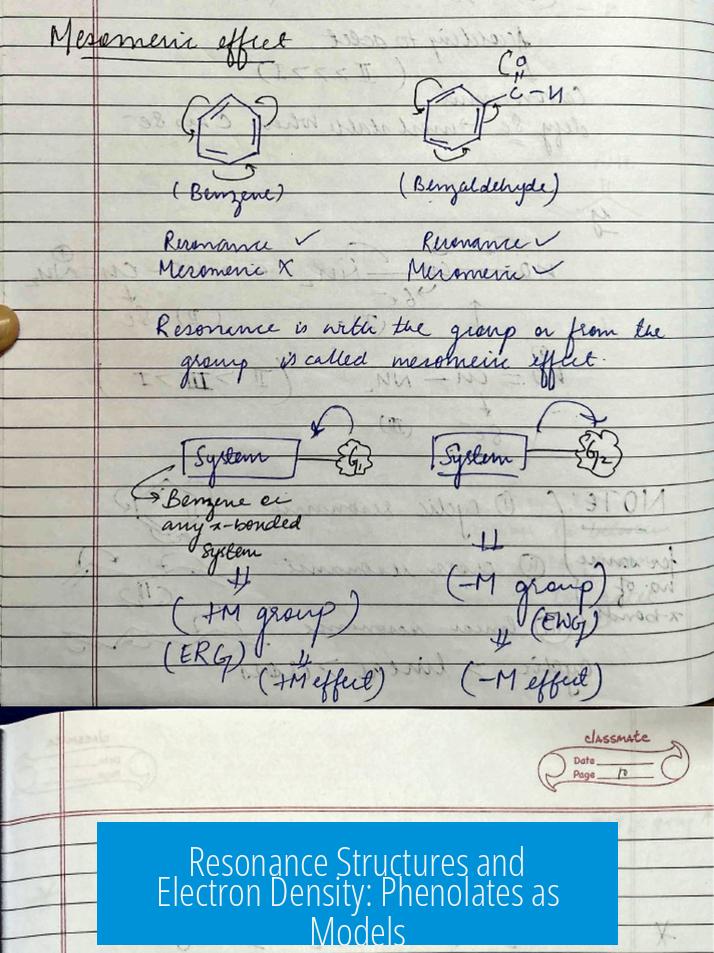
Resonance in phenols and phenolates offers insight. When phenol loses a proton, the phenolate ion forms. Its negative charge localizes chiefly on oxygen, stabilizing the conjugate base.
Key points:
- Resonance structures showing negative charge on oxygen are more stable.
- Structures localizing negative charge on ring carbons (especially meta carbons) are destabilized due to increased electron density there.
- This implies resonance electron donation at meta positions is not favorable.
For resorcinol, negative charge stabilization through resonance predominates on oxygen rather than ring carbons, showing limited mesomeric effect at meta sites.
Competition Between Multiple Hydroxyl Groups
Resorcinol contains two hydroxyl groups capable of resonance interaction. However, these groups compete for resonance structures that delocalize electron density.
- Only one hydroxyl or phenolate oxygen can effectively resonate with the ring at a time.
- This competition limits simultaneous resonance effects at multiple positions.
- The meta hydroxyl group competes with the phenolate oxygen, diminishing resonance donation from that hydroxyl at meta carbons.
This phenomenon further reduces the chance that a meta hydroxyl group experiences positive mesomeric resonance with the aromatic ring.
Implications from Acidity and pKa Data
Experimental acidity (pKa) values offer evidence for theoretical resonance descriptions.
- Phenolates with stabilized negative charge on oxygen have lower acidity.
- Resorcinol’s two hydroxyls affect acidity, but the meta hydroxyl does not significantly enhance resonance stabilization of the conjugate base.
- Observed pKa values correlate with dominant negative charge localization on oxygen rather than the ring carbons.
This indicates resonance effects involving the meta hydroxyl group do not significantly stabilize the conjugate base, implying a lack of positive mesomeric effect at meta position.
Misconceptions About Hydroxyl Groups Increasing Electron Density Everywhere
A common misunderstanding is that all hydroxyl groups increase electron density throughout the ring equally, reducing acidity at all positions.
Clarifications:
- Electron density distribution is complex and position-specific.
- Mesomeric (resonance) effects vary between ortho, meta, and para carbons.
- The negative charge in phenolates delocalizes with different intensities at different ring positions.
- Electron donation by hydroxy groups is not uniform; meta positions see negligible positive mesomeric contribution.
These points emphasize that resonance effects from hydroxy groups depend heavily on their location within the ring, modulating electronic properties differently.
Summary Table: Mesomeric Effect of Hydroxyl in Resorcinol
| Position | Mesomeric Effect of Hydroxyl | Electron Density Impact | Stabilization |
|---|---|---|---|
| Ortho | Positive (+M) | Increase in electron density | Resonance stabilizes ring positions |
| Para | Positive (+M) | Increase in electron density | Resonance stabilizes ring positions |
| Meta | No positive mesomeric effect; slight inductive withdrawal (-I) | Electron density not enhanced by resonance | Limited stabilizing resonance contribution |
Key Takeaways
- Resorcinol’s meta hydroxyl group does not exert a positive mesomeric effect on the benzene ring.
- Hydroxyl groups increase electron density mainly at ortho and para positions via resonance.
- Meta position primarily experiences inductive effects with no significant resonance electron donation.
- Negative charge in phenolates is mainly localized on oxygen, stabilizing conjugate bases more than any ring carbon position.
- Multiple hydroxyl groups in resorcinol compete for resonance interaction, limiting simultaneous positive resonance effects.
- Acidity data (pKa) supports the dominance of resonance effects centered on oxygen rather than meta ring carbons.
Does the meta hydroxyl group in resorcinol show a positive mesomeric effect?
No, the meta hydroxyl does not exhibit a positive mesomeric effect. Instead, it mainly shows an inductive electron withdrawing effect at that position, unlike ortho or para positions where resonance donation is significant.
How do resonance structures affect electron distribution in resorcinol?
Resonance structures where negative charge is localized on oxygen are stabilized. Carbon-localized negative charges are destabilized due to increased electron density from resonance donation. This limits the positive mesomeric effect of the meta hydroxyl.
What role do multiple hydroxyl groups play in resorcinol’s resonance?
The hydroxyl groups compete for resonance with the phenolate oxygen. The meta hydroxyl’s resonance ability is reduced because it cannot resonate simultaneously with the phenolate oxygen, weakening any positive mesomeric effect.
Does experimental pKa data support resonance interpretations for resorcinol?
Yes, pKa values suggest that resonance stabilization primarily involves negative charge localized on oxygen. This supports the conclusion that the meta hydroxyl group does not significantly stabilize via positive mesomeric effects.
Why do some people mistakenly think all hydroxyl groups increase electron density uniformly?
It’s a misconception. Electron donating or withdrawing effects depend on position and resonance. In resorcinol, meta hydroxyl groups withdraw electrons inductively rather than donating by resonance, resulting in complex charge distribution patterns.




Leave a Comment