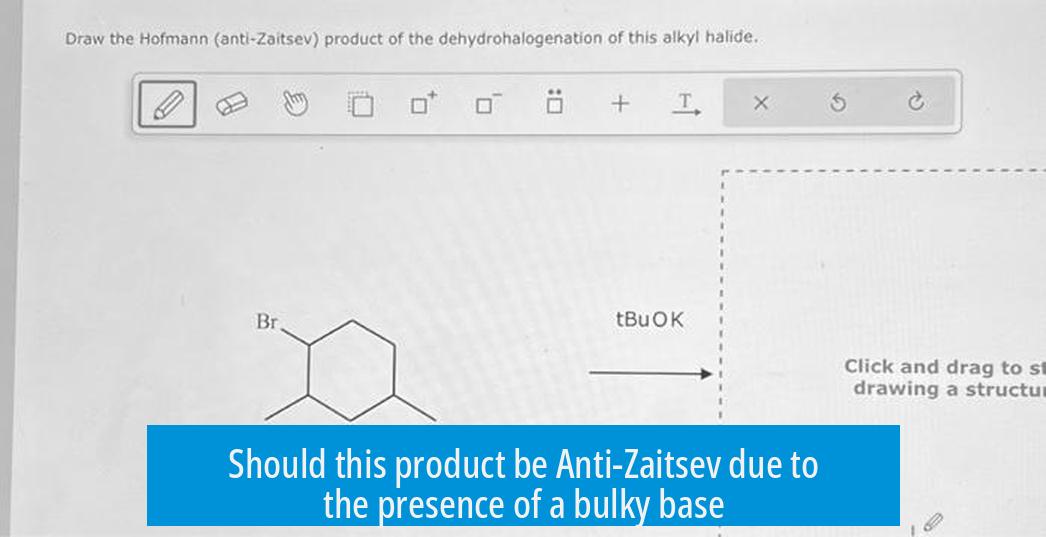
Shouldn’t this product be Anti-Zaitsev since the base is bulky?

The product of an elimination reaction is not always anti-Zaitsev even with a bulky base; reaction conditions, temperature, and thermodynamic stability often favor the Zaitsev (more substituted) product despite the steric bulk of the base.
Bulky Base and Expected Product Outcome
From an undergraduate perspective, bulky bases such as DBU, DBN, or KOtBu tend to favor the anti-Zaitsev (Hoffmann) product. This occurs because steric hindrance makes abstraction of the less hindered β-hydrogen easier. The anti-Zaitsev product typically forms faster, representing the kinetic product of the reaction.
Kinetic vs Thermodynamic Control
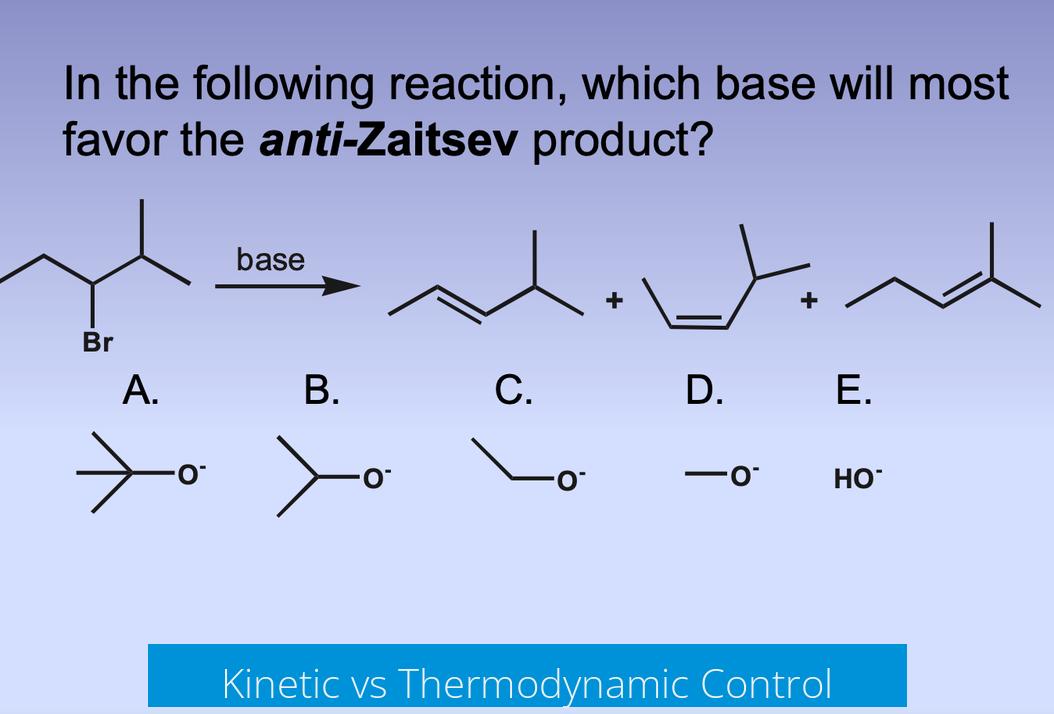
Elimination reactions can yield kinetic or thermodynamic products. The anti-Zaitsev product forms faster but is generally less stable. The Zaitsev product is more substituted and more stable, making it the thermodynamic product.
Higher temperatures supply energy to overcome activation barriers, allowing equilibration toward the thermodynamic Zaitsev product. Consequently, even with bulky bases, heating often shifts product distribution toward the more stable alkene.
Role of Reaction Conditions and Base Strength
- Strong bases like LDA at low temperature (-78°C) reliably give kinetic (anti-Zaitsev) products.
- Increasing temperature can lead to mixtures or favor thermodynamic products.
- KOtBu, less strong than LDA, may require heat to favor thermodynamic product formation despite its bulk.
Limitations of Sterics Alone
Steric effects do not always dominate selectivity. When β-protons are not significantly different in steric environment, the transition state leading to the thermodynamically stable Zaitsev product can be favored. Therefore, overall free energy considerations often outweigh steric hindrance in determining the product.
Equilibrium and Solvent Effects
In protic solvents, elimination can be reversible due to the presence of HBr. The system can equilibrate, favoring the more stable, typically Zaitsev product. Aprotic solvents can lock in the initially formed kinetic product, sometimes allowing the anti-Zaitsev product to predominate.
Summary of Key Points
- Bulky bases favor anti-Zaitsev products kinetically but not always thermodynamically.
- Higher temperatures promote formation of Zaitsev products by enabling equilibration.
- Steric hindrance is important but can be overridden by overall product stability.
- Reaction solvent and reversibility impact the final product distribution.
- Strong, non-nucleophilic bases at low temperature most reliably give anti-Zaitsev products.




Leave a Comment