Understanding SN1, SN2, E1, and E2 Mechanisms
SN1, SN2, E1, and E2 are fundamental organic reaction mechanisms that differ in how substitution and elimination happen based on substrate, nucleophile/base strength, and reaction conditions. These distinctions guide predicting products and their stereochemistry.
SN1 and E1 Mechanisms
SN1 and E1 proceed via a two-step process. Both form a carbocation intermediate after the leaving group departs. This intermediate allows for rearrangements like methyl or hydride shifts.
- Substrate: Favor tertiary haloalkanes, where carbocation formation is most stable.
- Nucleophile/Base: SN1 uses weak nucleophiles; E1 requires weak bases.
- Solvent: Polar protic solvents stabilize carbocations, favoring SN1.
- Product: E1 yields the more substituted alkene (Zaitsev product), with no Hoffmann product formation.
SN2 and E2 Mechanisms
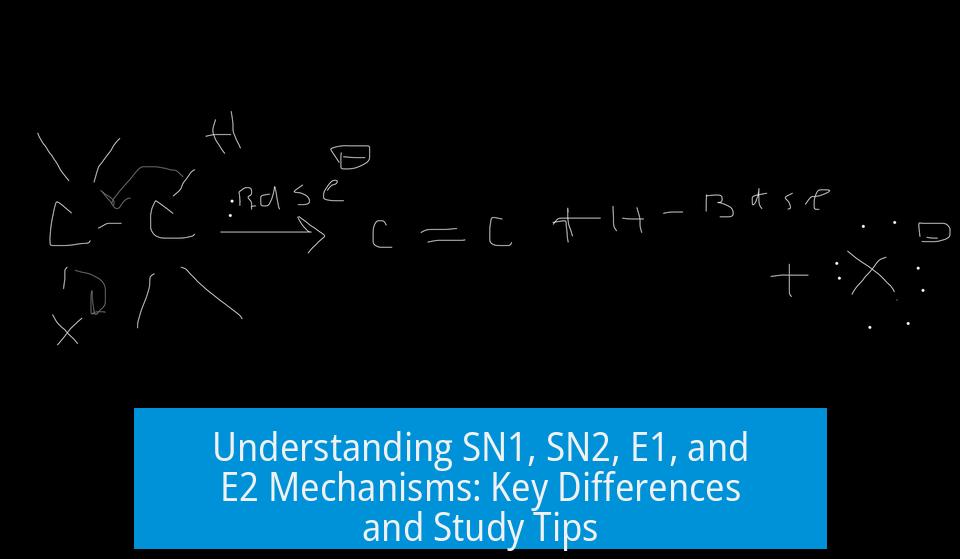
SN2 and E2 proceed via a concerted single-step process without intermediates. Both mechanisms happen simultaneously: bond-breaking and bond-forming occur in one step.
- Substrate: SN2 prefers methyl or primary substrates due to steric hindrance; E2 often occurs on tertiary substrates.
- Nucleophile/Base: SN2 requires a strong nucleophile; E2 demands a strong base.
- Solvent: Polar aprotic solvents facilitate SN2 by enhancing nucleophile strength.
- Outcome: SN2 inverts stereochemistry at chiral centers (Walden inversion). E2 eliminates a proton leading to alkene formation.
- Secondary Substrates: Can produce mixtures; E2 usually predominates with strong bases.
Substitution vs. Elimination
Substitution replaces the leaving group with a nucleophile, while elimination removes a proton and the leaving group to form a double bond.
In substitution, expect stereochemical inversion if the carbon is a chiral center. Elimination typically yields the more substituted alkene (Zaitsev product), except under special conditions.
Summary Table of Key Differences
| Mechanism | Steps | Intermediate | Substrate Preference | Nucleophile/Base | Solvent | Product Features |
|---|---|---|---|---|---|---|
| SN1 | 2-step | Carbocation | Tertiary | Weak nucleophile | Polar protic | Inversion + Racemization |
| SN2 | 1-step | None | Methyl/Primary | Strong nucleophile | Polar aprotic | Inversion (Walden) |
| E1 | 2-step | Carbocation | Tertiary | Weak base | Polar protic | Zaitsev alkene only |
| E2 | 1-step | None | Tertiary (often) | Strong base | Varies | Zaitsev & possible Hoffman |
Effective Study Tips
Master these mechanisms by practicing various exercises and checking solutions. Use video tutorials and PDFs for guided learning. Recognize patterns such as substrate type, nucleophile/base, and solvent to predict which mechanism dominates.
- Look for carbocation shifts (SN1/E1).
- Identify strong nucleophiles/bases for SN2/E2.
- Check solvent polarity (protic vs aprotic).
- Observe stereochemical changes.
Key Takeaways
- SN1/E1: Two-step, carbocation intermediate, weak nucleophiles/bases, favor tertiary substrates.
- SN2/E2: One-step, no intermediate, strong nucleophiles/bases, depends on substrate sterics.
- Solvents: Polar protic for SN1/E1; polar aprotic for SN2.
- Products: SN2 inverts stereochemistry; E1 gives Zaitsev alkenes; E2 can give Zaitsev or Hoffmann.
- Practice: Exercises and solution checking improve understanding and speed.
What indicates that a reaction follows an Sn1 or E1 mechanism?
Sn1 and E1 mechanisms form a carbocation intermediate. Look for methyl or hydride shifts. These shifts suggest the presence of a carbocation, pointing to Sn1 or E1 rather than Sn2 or E2.
How do nucleophile and base strength differ between Sn1/E1 and Sn2/E2?
Sn1 reactions use weak nucleophiles, while Sn2 uses strong nucleophiles. For elimination, E1 uses weak bases, and E2 uses strong bases. This helps distinguish between the pathways.
What substrate types favor Sn1 vs Sn2 or E1 vs E2?
- Sn1 and E1 prefer tertiary substrates due to carbocation stability.
- Sn2 favors methyl or primary substrates where backside attack is easier.
- E2 generally favors tertiary but can occur with secondary and primary under strong bases.
How can you tell the difference between substitution and elimination?
Substitution involves a nucleophile replacing a leaving group. Elimination removes a proton and the leaving group, forming a double bond. Substitution often inverts stereochemistry at chiral centers.
What solvent types favor Sn1 and Sn2 reactions?
Sn1 prefers polar protic solvents, which stabilize carbocations. Sn2 favors polar aprotic solvents, enhancing nucleophile strength and preventing carbocation formation.
Why doesn’t E1 produce Hoffmann products, unlike E2?
E1 always yields the Zaitsev product, the more substituted alkene. Hoffmann products don’t form because E1 proceeds via carbocation formation and elimination without base strength control that favors less substituted alkenes.



Leave a Comment