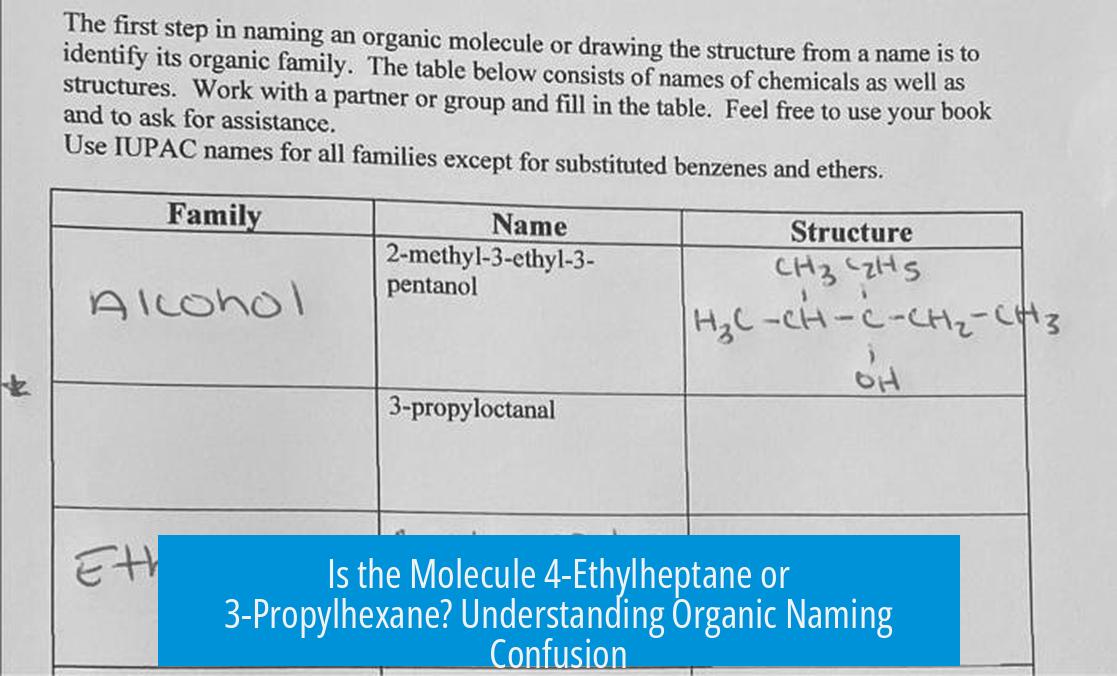
Is the Correct Name 4-Ethylheptane or 3-Propylhexane?
The correct IUPAC name of the molecule is 4-ethylheptane, not 3-propylhexane. This conclusion is based on applying the standard rules of nomenclature, particularly identifying the longest continuous carbon chain, which in this case contains seven carbons, resulting in the parent alkane heptane. The ethyl group on carbon 4 serves as the substituent. The alternative interpretation, 3-propylhexane, arises from an incorrect selection of the parent chain with only six carbons and a propyl substituent, which violates the requirement to name the molecule by its longest chain. Understanding this distinction eliminates confusion and ensures correct naming following IUPAC standards.
Longest Carbon Chain: The Core of Nomenclature
The first step in naming an alkane is finding the longest continuous carbon chain. This chain determines the base name of the compound. The parent chain must be a continuous, unbranched path of connected carbons.
- In this molecule, the longest continuous chain contains 7 carbon atoms, yielding the root name “heptane.”
- Misinterpreting the longest chain as containing only 6 carbons leads to the alternate, incorrect name using “hexane.”
The direction in which the chain is analyzed is crucial.
Why is Chain Selection Tricky?
The molecule may appear to have multiple paths. For example:
- Reading from left to right: may identify a 6-carbon chain.
- Reading from top right down to bottom right: recognizes a full 7-carbon chain.
A rigid left-to-right visual assessment often misses the actual longest chain.
Practical tip: One should either trace the carbon framework physically (with a pencil) or mentally without lifting the pencil between atoms. Whichever trace hits the highest number of carbons is the correct longest chain.
Position and Naming of Substituents
After identifying the parent chain, focus on naming substituents. A substituent is any group branching off the parent chain.
- The substituent in this case is an ethyl group (–CH2CH3).
- Its correct location is on carbon 4 of the heptane chain.
The combination results in the name: 4-ethylheptane.
Common Naming Pitfalls
Some may incorrectly identify the substituent as propyl at position 3 when choosing a hexane parent chain. This leads to the name 3-propylhexane.
- This name is incorrect because it does not reflect the longest carbon chain.
- The chain containing only six carbons is shorter than the actual seven-carbon chain.
- A substituent consisting of three carbons attached to a six-carbon chain is less prioritized than a substituent consisting of two carbons on a seven-carbon chain in IUPAC naming.
Correct nomenclature always prioritizes the longest continuous carbon chain as the parent structure, not what looks convenient at first glance.
How to Confirm the Correct Chain and Name
Follow this systematic approach to determine the correct IUPAC name:
- Identify all plausible continuous carbon chains. For this molecule, explore every path, including diagonals, zigzags, or turns.
- Count the number of carbons in each chain. The chain with the most carbons (7) is the parent chain.
- Number the parent chain from the end nearest the substituent. Here, numbering from one end places the ethyl group on carbon 4, the lowest possible number.
- Name the substituent groups according to their size. An ethyl group (two carbons) at position 4 is more accurate than labeling a propyl group at position 3 on a shorter chain.
Using these steps leads directly to the name 4-ethylheptane.
Table: Comparing Naming Options
| Aspect | 4-Ethylheptane | 3-Propylhexane (Incorrect) |
|---|---|---|
| Longest Chain Length | 7 carbons | 6 carbons |
| Parent Name | Heptane | Hexane |
| Substituent | Ethyl group at C-4 | Propyl group at C-3 |
| Consistency with IUPAC Rules | Follows longest chain rule, proper numbering | Violates longest chain rule |
Summary of Key Points
- The longest continuous carbon chain governs the parent name; here, it has 7 carbons, so the root name is heptane.
- The substituent is an ethyl group attached at carbon 4, resulting in “4-ethylheptane.”
- Choosing a shorter 6-carbon chain with a propyl substituent (3-propylhexane) is incorrect due to violation of IUPAC longest chain rules.
- To avoid errors, use a pencil-tracing method when analyzing chain length to ensure no chains are overlooked.
- Number the carbon chain to give substituents the lowest possible numbers.
Implications for Naming Organic Molecules
Correctly identifying the longest carbon chain prevents misnaming. Accurate naming is essential for clear communication in organic chemistry. It ensures consistency in databases, research, and educational contexts.
The example of choosing between 4-ethylheptane and 3-propylhexane illustrates the need for careful structural analysis rather than relying on superficial chain appearances.
Why is the molecule named 4-ethylheptane instead of 3-propylhexane?
The longest carbon chain contains seven carbons, making “heptane” the parent. The substituent is an ethyl group on carbon 4. Naming it 3-propylhexane ignores the actual longest chain, which is critical for correct nomenclature.
How can I correctly identify the longest carbon chain in this molecule?
You must trace the chain in all possible directions, not just left to right. Drawing the molecule and tracing a line without lifting your pencil helps find the longest continuous chain, which here is seven carbons long.
Why does the 3-propylhexane interpretation seem convincing at first?
Looking left to right, it appears there is a six-carbon chain with a three-carbon propyl group attached at carbon 3. However, this reading overlooks a longer seven-carbon chain that includes the substituent differently.
Could the substituent be considered a propyl group instead of an ethyl group?
No. The substituent is an ethyl group attached to the fourth carbon of the heptane chain. The propyl group interpretation arises from a mistaken longest chain count and is not consistent with the actual structure.
What practical advice helps avoid confusion in naming such molecules?
Always find the longest chain first by tracing all possible routes. Count the carbons carefully and avoid relying only on the diagram’s orientation. Mark substituents after confirming the parent chain’s length.



Leave a Comment