Stereochemistry – Why Are These 2 Compounds the Same?
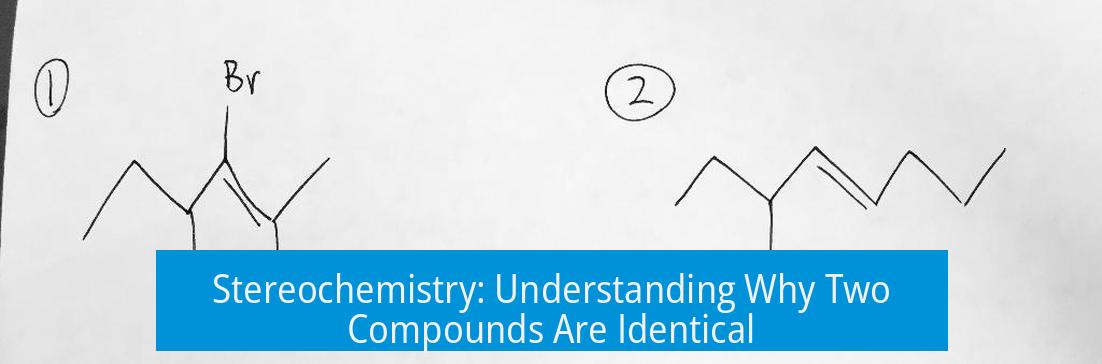
Two compounds that might look different at first glance can often be identical due to their stereochemical properties. They are the same when they have the same substituents arranged in the same three-dimensional configuration, making them superimposable despite different representations.
Understanding why two compounds with seemingly different drawings or orientations are actually identical hinges on several key concepts of stereochemistry. These include the use of physical models, superimposability, stereochemical configuration (R/S notation), rotation and swaps of substituents, and analogies such as Fischer projections. Exploring these topics clarifies why the two compounds in question are the same.
1. Visualization and Physical Models
Many learners struggle to visualize molecules in three dimensions. This difficulty can lead to confusion about whether two structures are truly different. Physical models come to the rescue here.
- Building models with common household items helps bridge the visualization gap. Toothpicks paired with playdough or pencils with wads of paper allow one to recreate molecular shapes and see spatial arrangements concretely.
- 3D ball-and-stick kits are highly recommended. These kits provide immediate insight into whether two compounds can be transformed into one another by rotation or flipping. They help demonstrate that what appears different on paper can be the same in space.
By physically manipulating these models, one can see that what looks like different orientations from flat drawings actually belong to the same compound.
2. Superimposability and Molecular Orientation
A molecule’s identity depends on whether it can be superimposed onto another by rotation or movement in three-dimensional space. Two compounds that appear different in pictures might be related simply by a spatial reorientation.
Key points to consider:
- The first compound might have the vinyl group pointing upward, while the second has the ethyl group in that direction.
- Rotating the second molecule until the vinyl group faces upward as in the first molecule can reveal the two molecules are identical.
- This superimposability is independent of the exact drawing or perspective. Just because the groups face different directions on paper doesn’t mean the molecules differ.
The analogy of scissors illustrates this well: a right-handed pair of scissors doesn’t become left-handed by simply turning it around. Orientation matters but does not change the fundamental nature of the object.
3. Stereochemical Configuration and Assignment
Stereochemical assignments (R/S) provide a systematic way to determine if two molecules are identical.
Considerations in this context:
- If both molecules have the same substituents bonded to the same carbon but differ in spatial arrangement, they could be enantiomers or identical.
- Checking the configuration reveals whether they match. If both have the same R or S configuration, they are the same molecule.
- For example, two compounds each have groups H, CH3, CH2CH3, and CH=CH2 bonded to a chiral center. If both have an R configuration at the stereocenter, they are identical, not mirror-image enantiomers.
Wedge notation helps confirm these assignments. When hydrogen is incorrectly shown on a solid wedge but should be dashed, the configuration reverses. Both compounds share (R,R) configuration, supporting their identity.
4. Rotations, Swaps, and Equivalent Transformations
There are practical ways to understand how two compounds relate by focusing on substituents’ positions and swaps.
Rotation-based view:
- Imagine holding the methyl group fixed at the back of the molecule.
- Rotate the other three substituents around like fan blades. A clockwise or counterclockwise move can convert one drawing into the other.
Swapping substituents:
- Perform two sequential swaps of groups. For instance, swapping vinyl (–CH=CH2) with ethyl (–CH2CH3), then vinyl with hydrogen (–H) can change one structure into the other.
- An even number of swaps implies no overall change in stereochemistry, meaning the two structures represent the same molecule.
This approach offers an operational way to verify identity beyond theoretical stereochemical assignments.
5. Fischer Projection Analogy
Fischer projections offer another perspective to understand the equivalency of these molecules.
- In Fischer projections, vertical chains face away and horizontal bonds face toward the viewer, simplifying stereochemistry visualization.
- Rotating substituents within the Fischer projection can clarify how different drawings represent the same stereochemistry.
- The analogy suggests that rotating three of the four substituents around a fixed position changes the molecule’s drawing but not the molecule itself.
Referring to educational resources or interactive graphics on the Fischer projection can further aid in visualizing these concepts.
Summary of Key Points
- Physical models help resolve visualization problems by providing tangible 3D structures.
- Superimposability confirms if two molecules are the same despite initial differences in orientation.
- Stereochemical configuration (R or S) confirms the identity of molecules with identical substituents.
- Rotation and swapping of substituents offer practical, straightforward methods to interconvert molecular drawings.
- Fischer projection analogies support understanding of stereochemical equivalencies across different representations.
Recognizing two compounds as the same stereochemically requires evaluating their 3D arrangement and substituent placement, not just their 2D depictions. By applying these principles, chemists avoid confusion and correctly identify molecular identities.
Why do these two compounds look different but are actually the same?
The two compounds differ in orientation, not structure. By rotating one molecule or swapping groups, you can superimpose it on the other. Their same stereochemistry confirms they are identical.
How can physical models help understand if two stereoisomers are the same?
Physical models show 3D arrangement clearly. Using ball-and-stick kits or simple items to build the molecules lets you see if the compounds overlap perfectly, proving they are the same.
What role does rotation around the methyl group play in identifying these compounds?
Holding the methyl group fixed and rotating other groups helps visualize equivalence. This rotation shows the groups align exactly, meaning the molecules are identical despite initial appearance.
How do swaps of substituents prove compound identity?
Swapping two pairs of substituents is an even number of exchanges, which preserves the molecule’s identity. Performing these swaps transforms one structure into the other, confirming they are the same compound.
Why does having the same R/S configuration mean the compounds are identical?
If both molecules have the same R or S stereochemical assignments, they cannot be mirror images or diastereomers. This means they are identical stereoisomers, confirming they are the same compound.




Leave a Comment