Understanding Syn vs. Anti Addition
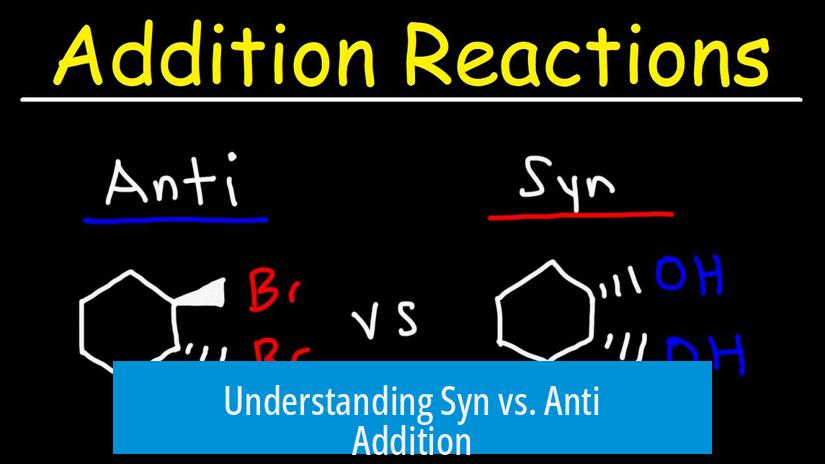
Syn addition involves adding two groups to the same side of a double bond, while anti addition places the groups on opposite sides. These modes define how substituents attach during reactions involving alkenes or alkynes.
1. Definitions
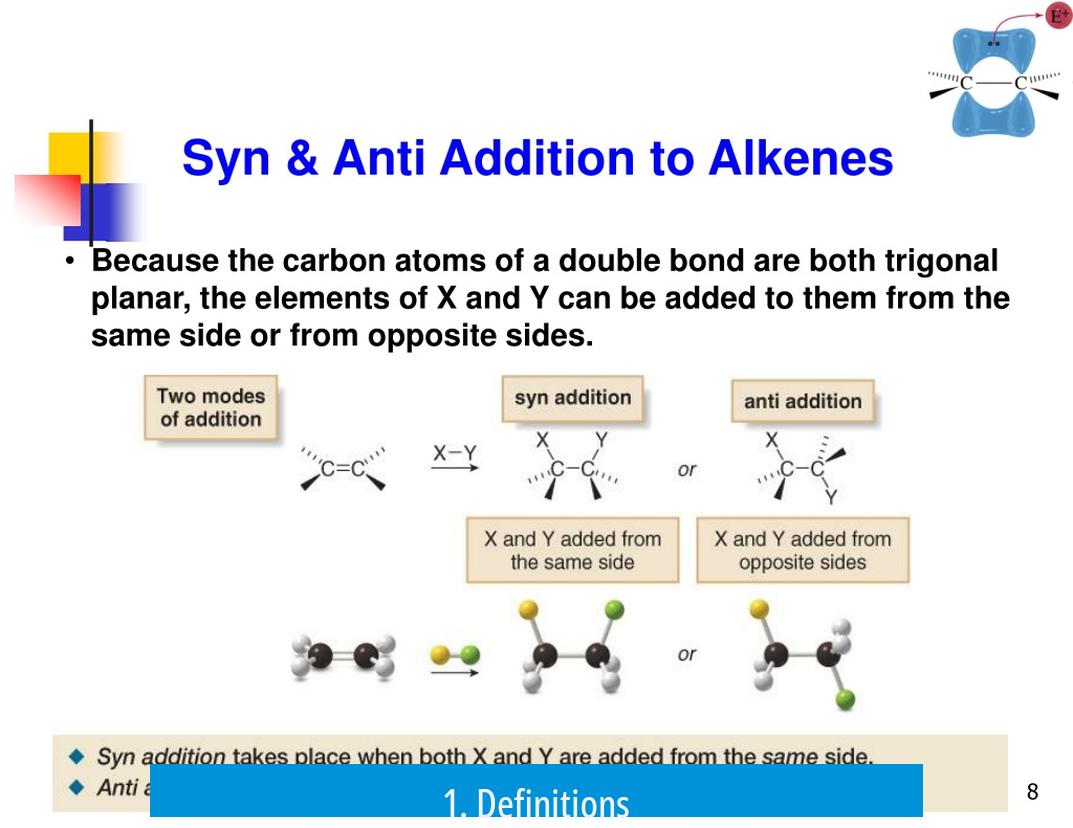
- Syn Addition: Both groups add to the same face of the double bond. In wedge-dash notation, this means both groups appear as wedges or both as dashes, indicating their orientation on the same side.
- Anti Addition: Groups add to opposite faces of the double bond. Here, one group is depicted as a wedge and the other as a dash, signaling opposite sides.
2. Relationship to Cis and Trans
Syn and anti additions refer specifically to the stereochemistry during the addition step and are independent from the terms cis and trans. Cis and trans describe the relative position of substituents around a double bond but do not define the addition process.
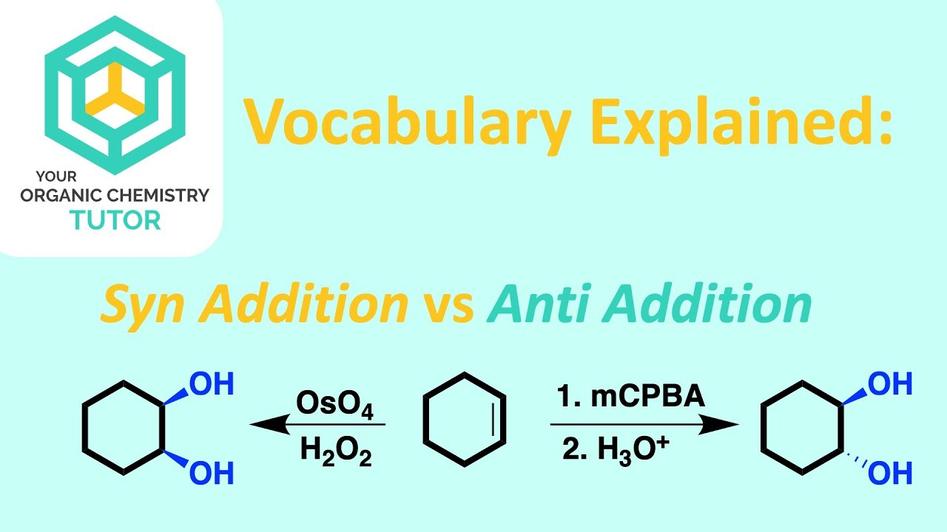
3. Visualizing Syn and Anti Addition
The wedge-dash notation helps distinguish these addition modes:

| Addition Type | Wedge-Dash Representation | Description |
|---|---|---|
| Syn Addition | Both wedges or both dashes | Groups add to the same side, creating stereochemical syn products. |
| Anti Addition | One wedge and one dash | Groups add to opposite sides, producing anti stereochemistry. |
4. Practical Importance
Synthetic chemists use syn and anti addition concepts to predict stereochemical outcomes of reactions like halogenation or hydroboration. Knowing whether an addition is syn or anti guides the design of molecules with desired 3D shapes.
5. Learning Aids
Visualizing these processes can be challenging. Using online videos and molecular models helps clarify the difference between syn and anti addition effectively.
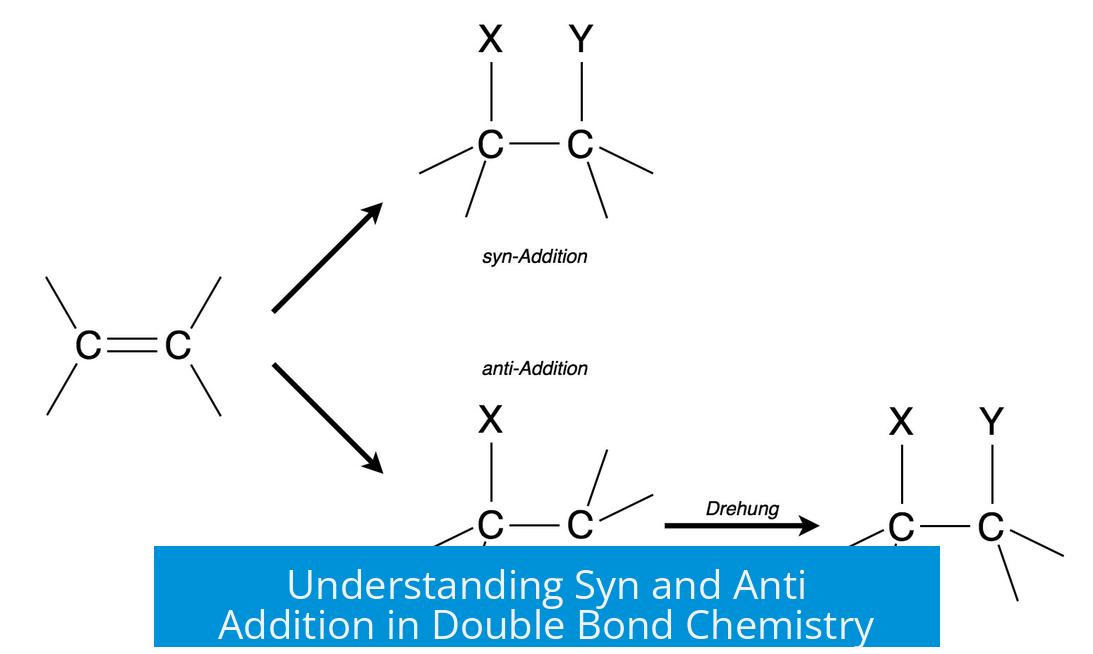
- Syn addition places groups on the same side of the double bond; anti addition places them on opposite sides.
- Syn/anti addition is distinct from cis/trans configuration about substituents.
- Wedge-dash notation is a key tool for representing syn and anti stereochemistry.
- Understanding addition modes is essential for predicting stereochemical outcomes in organic synthesis.
- External resources like videos and models aid in mastering these concepts.
Syn vs. Anti Addition: Cracking the Code of Double Bond Chemistry
Syn addition means groups add on the same side of a double bond, while anti addition means groups add on opposite sides. Sounds simple, right? But as with many chemistry concepts, the devil’s in the details. Let’s navigate the world of syn and anti addition, clear up common confusions, and learn how these terms relate to the widely known cis and trans structures.
What Exactly Are Syn and Anti Addition?
Imagine a double bond as a tiny stage. Syn and anti addition describe where the new “actors” (groups) enter the stage.
- Syn Addition places both groups on the same side of the double bond. In wedge-dash notation, both are either wedged (coming out toward you) or dashed (going back away from you). This means they share the same “face” of the stage.
- Anti Addition introduces the groups on opposite sides of the double bond. One group is wedged, and the other is dashed, showing they appear from different faces — like two actors entering the stage from opposite wings.
This is a subtle, yet crucial distinction. For instance, think of bromine adding across an alkene. Depending on the mechanism, bromine atoms can add syn or anti, affecting the 3D shape of the final molecule.
Is Syn/Anti the Same as Cis/Trans? Nope!
Here’s where many beginners stumble.
Syn and anti addition are NOT the same as cis and trans. Both describe spatial relationships, but they focus on different moments and molecular features:
- Syn and anti addition describe how new groups add to a double bond—simultaneously, during a reaction step.
- Cis and trans describe the relative positioning of two substituents on a double bond after the reaction is done.
Think of it like this: syn/anti is about the process of where groups land. Cis/trans is about the arrangement once everyone is in place. They’re independent descriptors.
Visualizing Syn and Anti Addition: Wedges and Dashes to the Rescue
If words alone confuse you, chemistry’s favorite 3D shorthand codes—wedges and dashes—lend a crystal-clear picture.
In wedge-dash diagrams:
- Syn addition: Both groups sit either on wedges or both on dashes, indicating they’re on the same face of the double bond.
- Anti addition: One group gets a wedge and the other a dash, showing opposite faces.
This neat notation helps chemists and students alike see the molecule in three dimensions without needing physical models.
For example, during the hydroxylation of an alkene by osmium tetroxide—a classic syn addition — both hydroxyl (OH) groups add from the same face, so both are wedged or dashed. On the flip side, in bromine addition, an anti addition mechanism often dominates, resulting in one bromine wedged and the other dashed.
Why Does It Matter? Real-World Benefits of Knowing Syn and Anti Addition
You might wonder, “Why bother remembering which side groups add on?” Here’s the scoop:
- Predicting products: Knowing syn vs. anti addition helps chemists predict 3D molecule shapes, key for understanding reactivity and interactions.
- Drug design: Some molecules need precise 3D shapes to work properly. Syn/anti addition knowledge guides creation of those shapes.
- Mechanism clues: Whether addition is syn or anti reveals clues about the reaction’s pathway (radical, ionic, or concerted).
In short, it’s like having the recipe details, not just the ingredients.
Pro Tip: Can’t Picture This? Use External Videos to Get That Visual Groove
If you’re struggling to picture who’s sitting where on the molecular stage, you’re not alone.
Helpful hint: The internet has tons of videos that animate syn and anti addition reactions vividly. Watching these can transform abstract notions into memorable scenes.
For example, YouTube channels dedicated to organic chemistry often use color-coded animations and 3D models to show syn vs. anti additions in real time. They illustrate how mechanisms differ and unique products form.
Comparison in a Nutshell
| Feature | Syn Addition | Anti Addition |
|---|---|---|
| Position of Added Groups | Both on the same side (both wedged or dashed) | Groups on opposite sides (one wedged, one dashed) |
| Relation to Cis/Trans | Independent; does not determine cis/trans | Independent; does not determine cis/trans |
| Typical Reaction Examples | Osmium tetroxide hydroxylation (syn diol formation) | Bromine addition across alkenes (anti-dihalide) |
Final Thoughts: Syn and Anti Addition Make Chemistry a 3D Puzzle Worth Solving
Syn and anti addition clarify tiny but vital spatial details in organic reactions. Getting them right is like nailing choreography in molecular ballroom dancing. Skip that step, and the whole performance could look off.
Understanding these terms empowers students and chemists to better predict reaction outcomes and design molecules with precision.
Still confused? Grab a model kit or check a video tutorial for that “aha” moment. Once syn and anti addition clicks, you’ll appreciate the elegance behind these labels. They’re more than jargon—they’re keys unlocking the intricate dance of atoms.
What is the key difference between syn and anti addition?
Syn addition adds groups to the same side of a double bond. Anti addition puts groups on opposite sides, with one wedged and one dashed to show this difference.
How does syn/anti addition relate to cis and trans isomerism?
Syn and anti addition are independent of cis and trans terms. Cis and trans describe the relationship between groups, not how they add during reactions.
How can I visually identify syn and anti addition using wedge-dash notation?
- Syn addition: both groups are either wedged or dashed (same side).
- Anti addition: one group is wedged, the other dashed (opposite sides).
Can syn and anti additions occur in the same molecule?
Yes, depending on the reaction mechanism, a molecule can undergo either syn or anti addition. The product stereochemistry depends on how groups add across the double bond.
Where can I find more visual aids to understand syn vs. anti addition?
Many online videos and tutorials explain syn and anti addition with animations. Watching these can improve your grasp of the concepts effectively.



Leave a Comment