Resonance Structures for Protonated Acetone
The resonance structures for protonated acetone arise after the oxygen atom donates a lone electron pair to bond with a proton, leading to a positively charged oxygen. This positive formal charge on oxygen is the basis for resonance in protonated acetone.
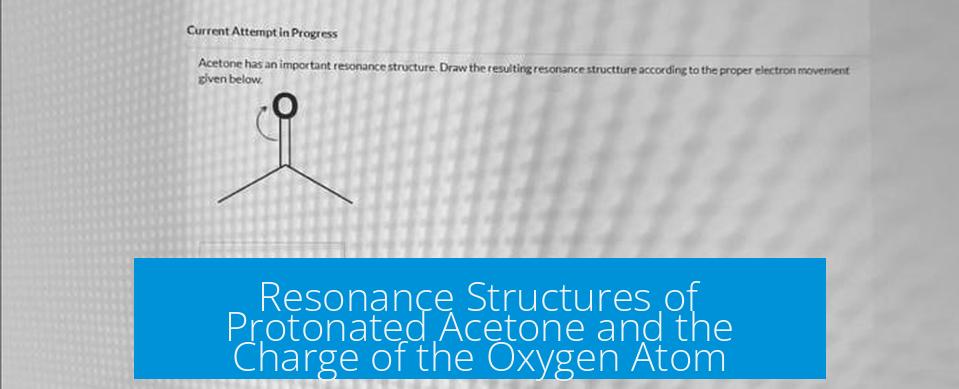
When acetone (CH3COCH3) is protonated, the lone pair on the carbonyl oxygen attacks the proton (H+), forming an oxonium ion. This changes the electron distribution and introduces resonance possibilities involving the oxygen and adjacent atoms.
Developing Resonance Structures
- The oxygen starts with two lone pairs and double bond to carbon.
- After protonation, oxygen shares one lone pair to bond with H+, gaining a positive charge.
- Because oxygen now has three bonds and only one lone pair, it carries a +1 formal charge.
- Resonance structures typically show electron delocalization shifting the positive charge and changing the double bond position between oxygen and carbon.
Resonance forms stabilize the protonated acetone by moving the positive charge through the molecule instead of localizing it solely on oxygen.
Why the Oxygen Atom Should Be Positively Charged After Protonation
Oxygen’s positive charge arises because it shares an electron pair with the proton, reducing the electrons it “owns.” The formal charge calculation counts the difference between valence electrons and those assigned to the atom in the molecule.
Since oxygen donates electron density to bond with H+, it loses some electron ownership. This results in a positive formal charge, not negative.
A negative charge on oxygen after protonation indicates an incorrect drawing or misunderstanding. Protonated oxygen atoms in carbonyl compounds do not carry negative charges.
No Oxygen Molecule Present
The discussion involves a single oxygen atom within acetone, not an oxygen molecule (O2). Interpretation errors often come from confusing atoms with molecules. Here, focus rests on the oxygen atom bonded within acetone’s structure.
Summary of Key Points
- Protonation of acetone involves the oxygen donating a lone pair to bond with a proton, creating a positively charged oxonium ion.
- Resonance structures distribute the positive charge throughout the molecule, enhancing stability.
- The oxygen in protonated acetone carries a positive formal charge, not negative.
- Negative charge on protonated oxygen indicates incorrect structure or drawing errors.
- This system contains a single oxygen atom, not an oxygen molecule.




Leave a Comment