What Does D or L-Sugar Really Mean?
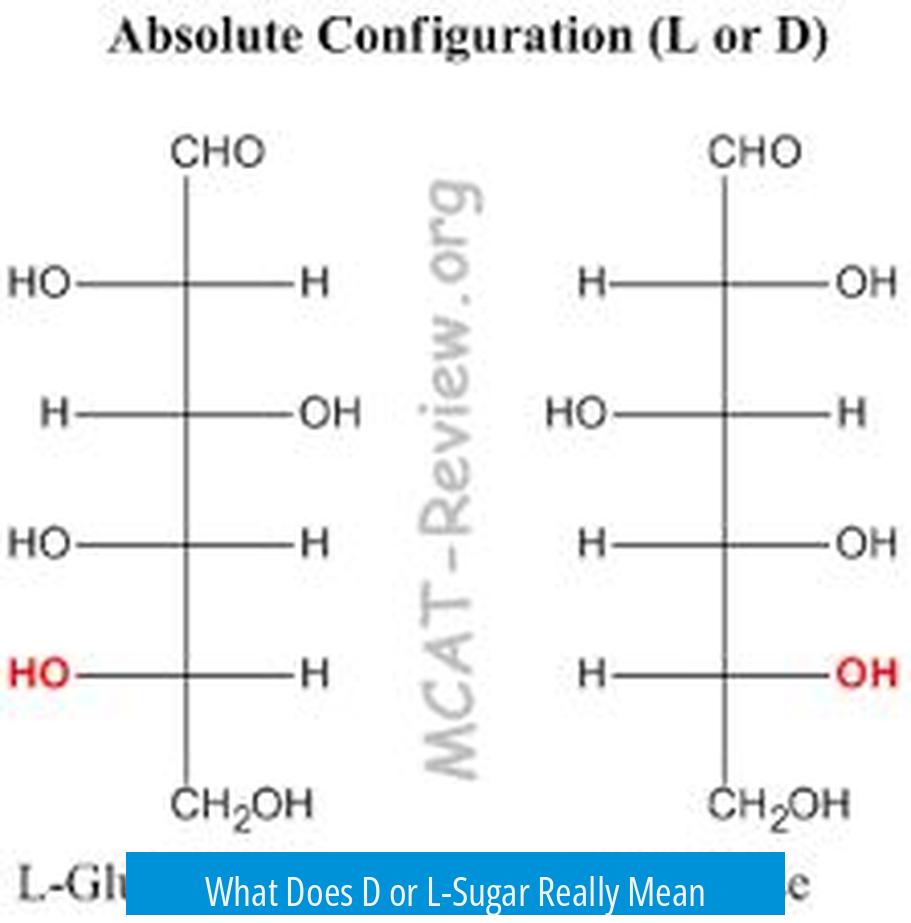
D- and L- sugars refer to the spatial arrangement of atoms around the highest numbered chiral center in a sugar molecule, indicating its stereochemistry and enantiomeric form. This designation comes from the orientation of the hydroxyl group on the chiral carbon furthest from the aldehyde or keto group when drawn as a Fischer projection.
Origin and Definition of D and L Nomenclature
The D/L system originates from Emil Fischer’s work on glucose structure. He assigned the absolute configuration of the highest-numbered chiral center by guesswork, later confirmed by X-ray crystallography. The notation indicates whether the hydroxyl group on this chiral center is oriented to the right (D) or left (L) in a Fischer projection.
- D- sugars have the hydroxyl group on the right side, generally corresponding to an R configuration at that carbon.
- L- sugars have it on the left side, generally corresponding to an S configuration.
This system is historical and primarily applies to naturally occurring compounds such as sugars and amino acids.
Molecular Structure and Chirality
The D/L descriptor applies only to the highest numbered chiral center. It does not define the full molecule’s overall chirality or the absolute configuration of all chiral centers.
For example, “R-glucose” does not exist as a whole molecule descriptor since R/S pertains only to individual stereocenters.
Relationship to Optical Rotation
Despite D-glucose being dextrorotatory (rotates plane-polarized light to the right), the D or L label does not reliably predict optical rotation direction. Enantiomers (D and L forms) rotate light in opposite directions, but the correlation is not strict.
Enantiomerism Between D and L Sugars
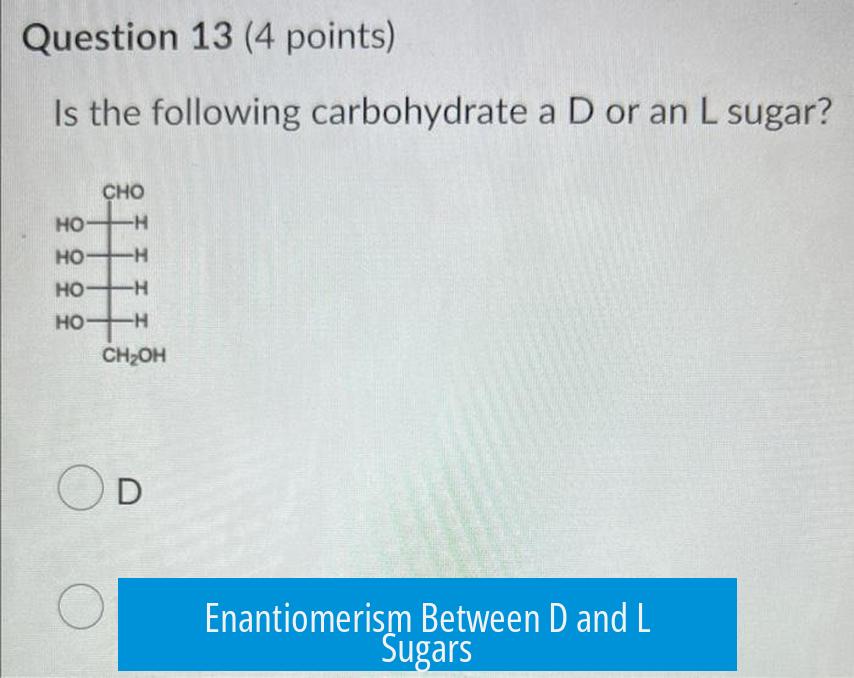
D and L sugars are enantiomers, meaning they are non-superimposable mirror images. Every chiral center in the L sugar is the mirror opposite of its D counterpart. This applies to sugar derivatives as well.
Biological Relevance
- Most naturally occurring sugars are D-forms.
- Conversely, most amino acids exist in the L-form.
- Biochemistry commonly uses D/L notation instead of R/S to describe sugars.
Important Clarifications
- D/L designation should not be confused with α/β anomers, which describe stereochemistry at the anomeric carbon.
- D/L indicates configuration, not preference or sweetness.
Key Takeaways
- D/L sugars describe stereochemistry at the highest numbered chiral center in Fischer projections.
- This system is historical and applies mostly to natural products.
- D and L sugars are enantiomers—mirror images with opposite chirality at all centers.
- D/L notation does not indicate optical rotation direction.
- Biochemical convention favors D/L for sugars and L for amino acids.



Leave a Comment