Welcome to the fascinating world of elements! Whether you’re a chemistry enthusiast or just curious about the building blocks of the universe, you’ve come to the right place. Today, we’re going to delve into the structure of atoms and explore how each element is arranged.
We’re going to start by looking at the element 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, which is made up of a combination of protons, neutrons, and electrons. An atom is the smallest particle of an element that still retains its characteristics. Its structure is made up of a nucleus, which contains the protons and neutrons, and an electron cloud, which consists of electrons.
The element 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is an example of a transition element, which are elements that fall in between groups on the periodic table. This element’s atomic number is 77, and its atomic weight is 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
The element 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is an example of a transition element as well. In this case, its atomic number is 73 and its atomic weight is 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
The element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is also a transition element. It has an atomic number of 46, an atomic weight of 102.9, and an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
The element 1s2 2s2 2p6 3s2 3p6 is a simpler element and it has an atomic number of 47, an atomic weight of 106.4, and an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
Finally, the element 1s 2s 2p 3s 3p has an atomic number of 2, an atomic weight of 4.0, and an electron configuration of 1s2 2s2 2p2.
This element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 has an atomic number of 78, an atomic weight of 195.1, and an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 5s2 4d10 5p6 6s2 4f14 5d7.
We hope you’ve enjoyed learning about these elements and their atomic structure. Whether you’re a student, professor, or just curious about chemistry, understanding elements is key to understanding the world around us. So now that you know the basics of electron configuration, go out and explore the mysterious world of the atomic structure!
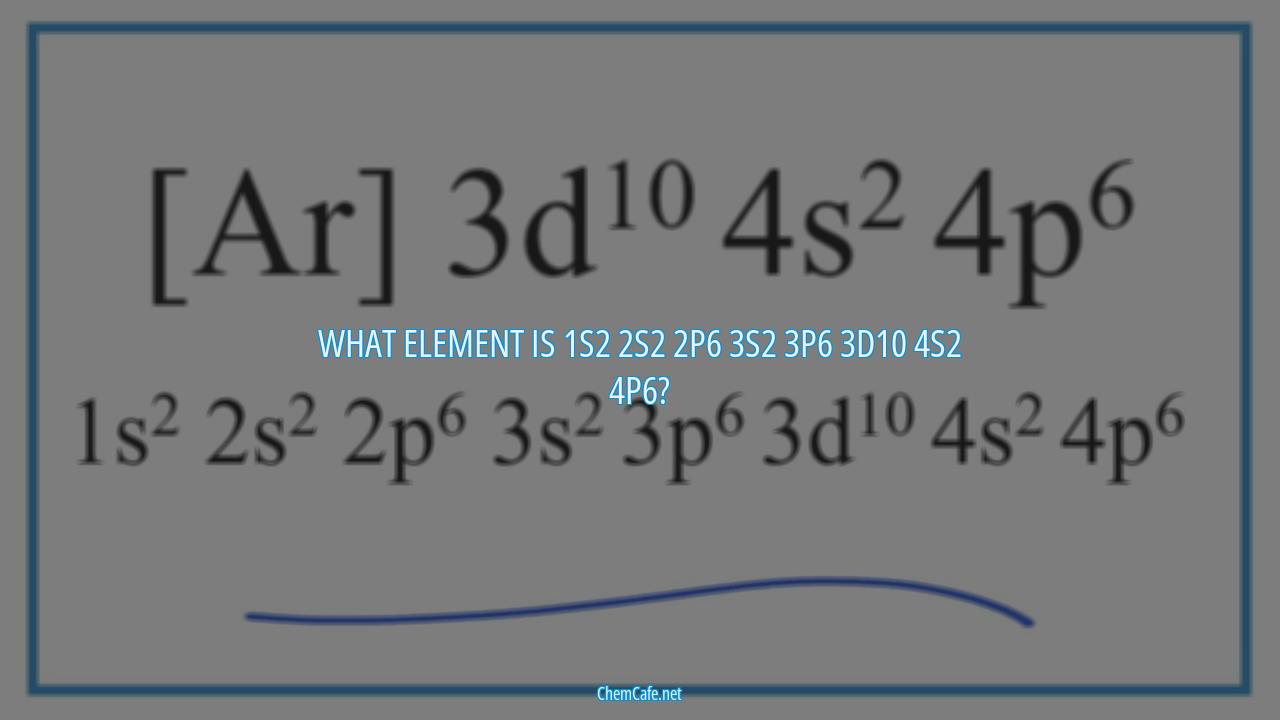
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
If you’re looking to identify an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, then you’re in the right place. This configuration is the electronic structure of four different elements, all of which are transition metals. These elements are iridium, tantalum, rhodium, and palladium. In this blog, we’ll discuss each of these elements in detail, including their atomic weights, atomic numbers, and groups.
Iridium
Iridium is the first element in this electron configuration. It has an atomic number of 77 and an atomic weight of 192.2. Iridium belongs to the group of transition elements, which are elements that have partially filled d orbitals in their outermost energy level. Iridium’s electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Tantalum
The next element in this electron configuration is tantalum. It has an atomic number of 73 and an atomic weight of 180.9. Like iridium, tantalum is a transition element that belongs to the group of transition elements. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Rhodium
Rhodium is the third element in this electron configuration. It has an atomic number of 46 and an atomic weight of 102.9. Rhodium is also a transition element and belongs to the group of transition elements. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Palladium
The last element in this electron configuration is palladium. It has an atomic number of 47 and an atomic weight of 106.4. Palladium is also a transition element and belongs to the group of transition elements. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 belongs to four different elements: iridium, tantalum, rhodium, and palladium. Each of these elements has its own unique atomic weight, atomic number, and group of elements. Knowing the electron configuration of an element can be helpful in identifying it, so we hope this information has been useful.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
When a scientist or anyone else talks about an element, they are referring to a single atom or a molecule that is composed of one type of atom. Every element has its own unique properties and characteristics based on the number of protons, neutrons, and electrons in the atom.
An element can be identified by its atomic number and its atomic weight, which are used to distinguish one element from another. The atomic number is the number of protons in an atom, while the atomic weight is the total number of protons and neutrons in an atom.
To identify an element, scientists often use a notation called the electron configuration. This notation uses the orbitals of the atom to identify the element. For example, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is for the element tantalum.
Tantalum
Tantalum is a transition metal that is part of the periodic table of elements. It has an atomic number of 73 and an atomic weight of 180.9. It is a hard, brittle metal that is resistant to corrosion and is used in electronics, jewelry, and alloys.
The electron configuration for tantalum is 1s2 2s2 2p6 3s2 3p6 4s2 3d3. This configuration shows that the atom has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, two electrons in the 4s orbital, and three electrons in the 3d orbital.
Iridium
Iridium is another transition metal found in the periodic table of elements. It has an atomic number of 77 and an atomic weight of 192.2. It is a very hard, brittle metal that is resistant to corrosion and is used in jewelry, electrical devices, and alloys.
The electron configuration for iridium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This configuration shows that the atom has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, two electrons in the 4s orbital, ten electrons in the 3d orbital, six electrons in the 4p orbital, two electrons in the 5s orbital, ten electrons in the 4d orbital, six electrons in the 5p orbital, two electrons in the 6s orbital, fourteen electrons in the 4f orbital, and seven electrons in the 5d orbital.
Rhodium
Rhodium is a transition metal found in the periodic table of elements. It has an atomic number of 46 and an atomic weight of 102.9. It is a very hard, brittle metal that is resistant to corrosion and is used in jewelry, electrical devices, and alloys.
The electron configuration for rhodium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7. This configuration shows that the atom has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, two electrons in the 4s orbital, ten electrons in the 3d orbital, six electrons in the 4p orbital, two electrons in the 5s orbital, and seven electrons in the 4d orbital.
Palladium
Palladium is a transition metal found in the periodic table of elements. It has an atomic number of 47 and an atomic weight of 106.4. It is a very hard, brittle metal that is resistant to corrosion and is used in jewelry, electrical devices, and alloys.
The electron configuration for palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8. This configuration shows that the atom has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, two electrons in the 4s orbital, ten electrons in the 3d orbital, six electrons in the 4p orbital, two electrons in the 5s orbital, and eight electrons in the 4d orbital.
In summary, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is for the element tantalum. This element is part of the transition metal group and has an atomic number of 73 and an atomic weight of 180.9. It is a hard, brittle metal that is resistant to corrosion and is used in electronics, jewelry, and alloys. Other transition metals with similar electron configurations include iridium, rhodium, and palladium.
Understanding the electron configuration of an element is an important part of understanding the properties of that element. By knowing the electron configuration, scientists can identify elements and predict the properties of those elements. With this knowledge, scientists can create materials with specific characteristics, such as metals that are resistant to corrosion or electrical devices that are more efficient.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
If you’re trying to figure out what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10, you’ve come to the right place. This is the electron configuration of three elements: tantalum, iridium, and radium. Here, we’ll discuss the basics of each element and provide some interesting facts about them.
Tantalum
Tantalum has the atomic number of 73, meaning it has 73 protons. It has an atomic weight of 180.9 and is a transition element. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Tantalum is a grey, hard, and heavy metal with a melting point of 3017°C. It is found in minerals such as columbite, tantalite, and euxenite. It is often used in electronics due to its ability to store electricity. It is also used in medical equipment and jewelry.
Iridium
Iridium has the atomic number of 77, meaning it has 77 protons. It has an atomic weight of 192.2 and is a transition element. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Iridium is a silvery-white, hard, and brittle metal. It has a melting point of 2410°C and a boiling point of 4428°C. It is found in two minerals: iridosmine and osmiridium. It is often used in hardening alloys, electrical contacts, and spark plugs. It is also used in jewelry and other decoration items.
Radium
Radium has the atomic number of 88, meaning it has 88 protons. It has an atomic weight of 226.0 and is a member of the alkaline earth metals. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2.
Radium is a radioactive, silvery-white metal. It has a melting point of 700°C and a boiling point of 1140°C. It is often used in medical treatments, dating rocks, and producing energy. It is also used in producing fluorescent dyes and in the manufacture of antifriction alloys.
So, to answer the question: what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10? The answer is tantalum, iridium, and radium. These elements have different applications and are important components of the periodic table. We hope this article has been helpful in understanding the electron configurations of these elements.
What element is 1s2 2s2 2p6 3s2 3p6?
Have you ever wondered what the element is with an electron configuration of 1s2 2s2 2p6 3s2 3p6? Have you been stuck trying to figure out which chemical element it is? Well, don’t worry, we’re here to help!
First of all, it is important to understand what an electron configuration is. Electron configuration is the arrangement of electrons in an atom or molecule. The electrons are arranged in different energy levels or shells, and each shell can hold a certain number of electrons. The 1s2 2s2 2p6 3s2 3p6 configuration is a way of representing the electrons in an atom or molecule.
So, what is the element with an electron configuration of 1s2 2s2 2p6 3s2 3p6? The answer is chlorine. Chlorine is a halogen and belongs to group 17 of the periodic table. It is a non-metal and has an atomic number of 17.
Chlorine has an atomic weight of 35.453 and is mainly used for bleaching, disinfecting, and water purification. Its atomic symbol is Cl and it has three naturally occurring isotopes. Chlorine is a pale greenish-yellow gas at room temperature, and it has a pungent odor.
What is Group 4A on the periodic table?
Group 4A (or IVA) of the periodic table includes the nonmetal carbon (C), the metalloids silicon (Si) and germanium (Ge), the metals tin (Sn) and lead (Pb), and the yet-unnamed artificially-produced element ununquadium (Uuq).
Group 4A elements are known as the carbon group and have similar chemical properties. These elements are characterized by having four valence electrons in their outermost shell. The elements in this group are all non-metals, except tin and lead.
What element is 1s2 2s2 2p3?
The element with an electron configuration of 1s2 2s2 2p3 is nitrogen (N). Nitrogen is a non-metal and belongs to group 15 of the periodic table. It has an atomic number of 7 and an atomic weight of 14.0067. Nitrogen is a colorless, odorless gas at room temperature, and is the most abundant element in the atmosphere.
Nitrogen has three naturally occurring isotopes, and it is an essential component of proteins and nucleic acids. Nitrogen is also used in fertilizers, explosives, and for industrial purposes.
What is the element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 belongs to the element titanium (Ti). Titanium is a transition metal and belongs to group 4 of the periodic table. It has an atomic number of 22 and an atomic weight of 47.867. Titanium is a silvery-gray metal at room temperature and is widely used in the aerospace industry.
Titanium has five naturally occurring isotopes and is highly resistant to corrosion. It is used in medical implants, aircraft engines, and jewelry.
We hope this article has been helpful in understanding the element with an electron configuration of 1s2 2s2 2p6 3s2 3p6. If you have any further questions, please feel free to contact us.
What is the element of 1s 2s 2p 3s 3p?
The element of 1s 2s 2p 3s 3p is chlorine (Cl). Chlorine is an element located in the periodic table in period 3, group 17. It has seven valence electrons and its electron configuration is 1s22s22p63s23p5.
The Electron Configurations of Neutral Atoms
The electron configuration of an atom is a representation of the arrangement of electrons in the orbitals of an atom. It is written in terms of the principal quantum number (n), the angular momentum quantum number (l), and the magnetic quantum number (m).
The first row of the periodic table contains two elements, hydrogen (H) and helium (He). Two electrons can go into the 1s subshell, and no more electrons can go into the 1s subshell since it is filled. This is followed by the second row elements, starting with lithium (Li) through neon (Ne). Two electrons can go into the 2s subshell, and six electrons can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell.
Chlorine’s Electron Configuration
Chlorine has two valence electrons in the 2s subshell and five valence electrons in the 2p subshell. This means that its electron configuration is 1s22s22p63s23p5. This is the electron configuration of neutral chlorine atoms.
Chlorine in a Chemical Reaction
Chlorine is a very reactive element and is essential for life. In a chemical reaction, chlorine can either gain or lose electrons to form chlorine ions. If chlorine gains one electron, it will form a chlorine anion with a -1 charge. If it loses one electron, it will form a chlorine cation with a +1 charge. Chlorine can also form covalent bonds with other elements, such as hydrogen.
In conclusion, the element of 1s 2s 2p 3s 3p is chlorine. Chlorine has seven valence electrons and its electron configuration is 1s22s22p63s23p5. Chlorine is a very reactive element and is essential for life. It can gain or lose electrons to form ions or form covalent bonds with other elements.
What is this element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
If you’re familiar with the periodic table of elements, you might have noticed that each element has a unique configuration of electrons. This electron configuration is a way of representing the energy levels and orbitals of an atom. It is often represented by a series of numbers and letters, such as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3.
The meaning of this particular electron configuration can be broken down into its component parts. The first part, 1s2, represents the innermost energy level and the number of electrons in the s-orbital. In this case, there are two electrons in the 1s orbital. The next part, 2s2, indicates that there are two electrons in the second energy level and the s-orbital. This means that there are two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5).
The 3s2 3p6 4s2 3d10 4p3 part of the electron configuration indicates that there are two electrons in the third energy level and the s-orbital (3s2), six electrons in the third energy level and the p-orbital (3p6), two electrons in the fourth energy level and the s-orbital (4s2), ten electrons in the fourth energy level and the d-orbital (3d10), and three electrons in the fourth energy level and the p-orbital (4p3). This means that the atom has seven valence electrons in total.
Examples of Elements with 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 Electron Configuration
There are several elements that have the 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 electron configuration. This includes elements from the transition metals group such as Rhodium (Rh), Palladium (Pd), and Iridium (Ir).
Rhodium
Rhodium is a transition metal located on the periodic table with the atomic number 46 and atomic weight 102.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Palladium
Palladium is another transition metal located on the periodic table with the atomic number 47 and atomic weight 106.4. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
Iridium
Iridium is a transition metal located on the periodic table with the atomic number 77 and atomic weight 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 indicates that there is a total of seven valence electrons. This configuration is shared by several elements from the transition metals group, including Rhodium (Rh), Palladium (Pd), and Iridium (Ir). Knowing the electron configuration of an element can help you understand its properties and behavior.



Leave a Comment