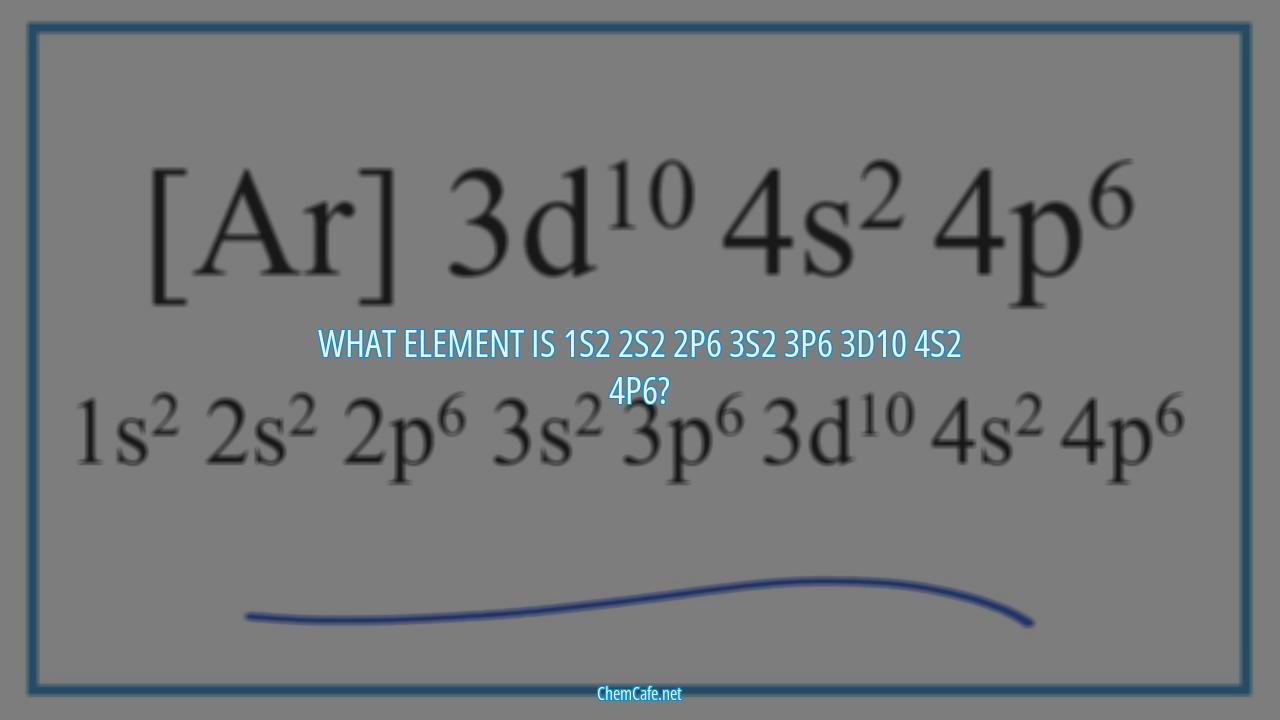
Are you looking to find out what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6? Have you ever wondered what element has the atomic number of 46, 47, 73, or 77? If so, you’ve come to the right place! In this blog post, we will discuss the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 and its different variations.
First, let’s start off by discussing the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. This element is iridium, which has an atomic number of 77 and an atomic weight of 192.2. Iridium is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
The element of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is tantalum, which has an atomic number of 73 and an atomic weight of 180.9. Tantalum is also a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
The element of 1s 2s 2p 3s 3p is palladium, which has an atomic number of 46 and an atomic weight of 102.9. Palladium is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d7.
The element of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is rhodium, which has an atomic number of 47 and an atomic weight of 106.4. Rhodium is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
Finally, the element of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is also rhodium. Rhodium has an atomic number of 47 and an atomic weight of 106.4. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3.
As you can see, there are several different elements and variations that are associated with the 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 configuration. Knowing the atomic number and weight of these elements can help you identify them and understand their properties. Now that you have a better understanding of the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, you can confidently explore the world of chemistry!
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is iridium. Iridium is a transition metal and is the second-densest element on the periodic table. It is a hard, brittle, and silvery-white metal that is highly resistant to corrosion. Iridium is one of the least reactive elements, making it an ideal material for use in catalytic converters, electrical contacts, and spark plugs.
Atomic Properties of Iridium
Iridium has an atomic number of 77, making it the 77th element on the periodic table. Its atomic weight is 192.2, and it has a density of 22.56 g/cm3. It is a relatively rare element, making up about 0.001 parts per million of the Earth’s crust.
Where Is Iridium Found?
Iridium is found in small amounts in meteorites and in minerals such as iridosmine and pegmatite. It is also found in small amounts in the Earth’s crust, but it is not commercially mined. Instead, it is usually recovered as a by-product of platinum and other metals mined in South Africa and Russia.
Uses of Iridium
Iridium is used in a variety of applications, including catalytic converters, electrical contacts, spark plugs, and jewelry. It is also used in the production of specialized alloys, such as high-temperature superalloys. These alloys are used in aircraft engines, gas turbines, and other high-temperature applications.
Health and Safety Considerations
Iridium is considered to be a non-toxic element, and it does not pose any health or safety risks. However, it is important to handle iridium with care, as it is a brittle metal that can shatter if handled improperly. It is also important to wear protective clothing and safety goggles when handling iridium.
Iridium is an important transition metal with a variety of uses, ranging from catalytic converters to electrical contacts. It has an atomic number of 77, an atomic weight of 192.2, and a density of 22.56 g/cm3. It is found in small amounts in meteorites and minerals, and it is usually recovered as a by-product of platinum and other metals. Iridium is considered to be a non-toxic element and does not pose any health or safety risks, but it is important to handle it with care.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
Atoms are made up of protons, neutrons, and electrons. Each element has a unique set of these particles, which are arranged in a particular way. The arrangement of these particles can be described using a notation called the electron configuration. The electron configuration of an element is a tool used to describe the orbitals occupied by the electrons of an atom.
The electron configuration of an element is written as a sequence of numbers and letters, which indicate the number of electrons in each orbital. For example, the electron configuration for the element tantalum is written as 1s2 2s2 2p6 3s2 3p6 4s2 3d3. This notation indicates that the element tantalum has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, two electrons in the 4s orbital, and three electrons in the 3d orbital.
Ta Name of Element : Tantalum
Tantalum is a transition element with an atomic number of 73 and an atomic weight of 180.9. It is a silvery-gray metal that is highly resistant to corrosion and has a melting point of 3017°C. It is a member of the transition element group, which is characterized by having partially filled d orbitals in their electron configuration. The electron configuration of tantalum is 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
Ir Name of Element : Iridium
Iridium is a transition element with an atomic number of 77 and an atomic weight of 192.2. It is a silvery-white metal that is highly resistant to corrosion and has a melting point of 2410°C. It is a member of the transition element group, which is characterized by having partially filled d orbitals in their electron configuration. The electron configuration of iridium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Rh Name of Element : Rhodium
Rhodium is a transition element with an atomic number of 46 and an atomic weight of 102.9. It is a silvery-white metal that is highly resistant to corrosion and has a melting point of 1963°C. It is a member of the transition element group, which is characterized by having partially filled d orbitals in their electron configuration. The electron configuration of rhodium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Pd Name of Element : Palladium
Palladium is a transition element with an atomic number of 47 and an atomic weight of 106.4. It is a silvery-white metal that is highly resistant to corrosion and has a melting point of 1552°C. It is a member of the transition element group, which is characterized by having partially filled d orbitals in their electron configuration. The electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
The electron configuration of an element is an important tool for understanding the chemical and physical properties of an element. By understanding the electron configuration of an element, chemists and physicists can predict how an element will react with other elements and how it will behave in different environments. Knowing the electron configuration of an element is also important for understanding its position in the periodic table.
In conclusion, 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is the electron configuration for the element tantalum. Tantalum is a transition element with an atomic number of 73 and an atomic weight of 180.9. It is a silvery-gray metal that is highly resistant to corrosion and has a melting point of 3017°C. It is a member of the transition element group, which is characterized by having partially filled d orbitals in their electron configuration.
What is the element of 1s 2s 2p 3s 3p?
The element of 1s 2s 2p 3s 3p is the atomic configuration of a neutral atom. It is used to describe the location of electrons in an atom’s outermost energy level. This is known as the valence shell and it is the most important part of an atom’s structure.
The 1s 2s 2p 3s 3p element configuration is made up of four parts: 1s, 2s, 2p, and 3s. The 1s is the first shell of electrons surrounding the nucleus. It can contain a maximum of two electrons. The 2s and 2p shells contain four electrons each and the 3s shell contains two electrons.
The 1s 2s 2p 3s 3p element configuration is used to represent an atom’s valence shell, or the most outermost energy level. This is because the valence shell is the only shell that can interact with other atoms and is responsible for an atom’s chemical properties.
When looking at the 1s 2s 2p 3s 3p configuration for a given element, it is important to remember that the number of electrons in each shell is always denoted by a superscript. For example, the element A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). The complete configuration for element A is thus 2s22p5. It has 2 + 5 = 7 valence electrons.
Element B
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). The complete configuration for element B is 3s2.
Element C
Element C is located in Period 5, the 1st position in 5s-block. This means that there is only one valence electron in 5s (5s1). The complete configuration for element C is 5s1.
Filling the Subshells
This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell. The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell.
Element Configuration of Chlorine
As an example, let’s look at the element chlorine. Chlorine has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5.
The 1s 2s 2p 3s 3p element configuration is an important concept in the study of atomic structure. It is used to represent an atom’s valence shell, which is responsible for its chemical properties. By understanding the 1s 2s 2p 3s 3p configuration of a given element, it is possible to gain insight into its behavior and reactivity.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
Atoms are composed of protons, neutrons, and electrons. The number of each type of particle in the atom is determined by its atomic number and its mass number. The atomic number is the number of protons in an atom and the mass number is the total number of protons and neutrons in an atom.
An atom’s electron configuration is an arrangement of electrons in an atom according to the subshells and shells of the atom. The electron configuration of an atom consists of a series of numbers followed by letters. For example, the electron configuration of an atom with the atomic number of 6 is 1s2 2s2 2p6.
The electron configuration of an atom can be used to identify the element. For example, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is the electron configuration of the element tantalum. This means that tantalum has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5).
The element iridium also has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10. However, the element iridium has different valence electrons than tantalum. This is because iridium is located in a different period of the periodic table than tantalum.
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). In the case of iridium, this is the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This means that iridium has two valence electrons in 3s (3s2) and five valence electrons in 3p (3p5).
Similarly, element C is located in Period 5, the 1st position in 5s-block. This means that there is only one valence electron in 5s (5s1). In the case of iridium, this is the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This means that iridium has one valence electron in 5s (5s1).
Tantalum
Name of Element : Tantalum
Atomic Weight : 180.9
Atomic Number : 73
Group : Transition Elements
Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10
Iridium
Name of Element : Iridium
Atomic Weight : 192.2
Atomic Number : 77
Group : Transition Elements
Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is the electron configuration of two transition elements: tantalum and iridium. Tantalum has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5), while iridium has two valence electrons in 3s (3s2) and five valence electrons in 3p (3p5). Additionally, iridium has one valence electron in 5s (5s1). Knowing the electron configuration of an element can be helpful in identifying its properties and understanding its behavior in chemical reactions.
What element is 1s2 2s2 2p6 3s2 3p6?
When it comes to understanding the periodic table, it can be difficult to know what element each electron configuration represents without a bit of practice and guidance. In this article, we’ll look at the element 1s2 2s2 2p6 3s2 3p6 and explain what it is, what group it belongs to, and what its name is.
1s2 2s2 2p6 3s2 3p6: The Element
The electron configuration 1s2 2s2 2p6 3s2 3p6 represents an element that belongs to group 17 on the periodic table, also known as the halogen group. This element is chlorine, which has an atomic number of 17 and an atomic mass of 35.45.
Chlorine: Properties and Uses
Chlorine is a yellowish-green gas at room temperature, and is the second-lightest halogen, making it one of the most reactive elements on the periodic table. It is used in a variety of applications, including water purification, bleaching, and disinfection. Chlorine is also used in the production of many everyday products, such as plastics, solvents, and pesticides.
Other Elements with 1s2 2s2 2p6 3s2 3p6 Configuration
In addition to chlorine, there are a few other elements that have an electron configuration of 1s2 2s2 2p6 3s2 3p6. These elements include titanium (Ti), neon (Ne), and ununquadium (Uuq).
Titanium (Ti) is a transition metal belonging to group 4A on the periodic table. It is a light gray metal with a high melting point, and is used in many everyday products, such as jewelry, medical implants, and aircraft parts.
Neon (Ne) is a noble gas with an atomic number of 10. It is colorless, odorless, and tasteless, and is used in neon lighting, fluorescent lamps, and lasers.
Ununquadium (Uuq) is an artificially-produced element with an atomic number of 114. It is highly radioactive, with a half-life of less than one millisecond.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 represents the element chlorine, which belongs to group 17 on the periodic table. It is a yellowish-green gas at room temperature and is used in a variety of applications, including water purification, bleaching, and disinfection. Other elements with this electron configuration include titanium (Ti), neon (Ne), and ununquadium (Uuq).
What is this element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Atoms are made up of protons, neutrons, and electrons. The electrons are organized into shells and subshells, and these shells and subshells are identified by their electron configuration. This is a notation system of writing down the number of electrons in each shell and subshell of an atom.
When looking at the electron configuration of an atom, the number of electrons in each shell and subshell is written in the form of an orbital diagram. The electron configuration of an element is written in the form of the symbol of the element followed by the orbital diagram.
For example, the electron configuration of the element Rhodium is written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. This means that Rhodium has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). Answer: 2s22p5. It has 2 + 5 = 7 valence electrons.
Element B
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Answer: 3s2. Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Answer: 5s1.
Rhodium
Rhodium is a transition element located in Group 9 of the periodic table. It has an atomic number of 46 and an atomic weight of 102.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7. This means that Rhodium has two valence electrons in 4s (4s2) and seven valence electrons in 4p (4p7).
Palladium
Palladium is a transition element located in Group 10 of the periodic table. It has an atomic number of 47 and an atomic weight of 106.4. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8. This means that Palladium has two valence electrons in 4s (4s2) and eight valence electrons in 4p (4p8).
Iridium
Iridium is a transition element located in Group 11 of the periodic table. It has an atomic number of 77 and an atomic weight of 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This means that Iridium has two valence electrons in 5s (5s2) and seven valence electrons in 5p (5p7).
The electron configuration of an element is an important factor in determining its reactivity and other chemical properties. Knowing the electron configuration of an element can help scientists and engineers to predict how the element will behave in different circumstances. This knowledge is essential for designing new products, developing new technologies, and understanding how different elements interact with each other.




Leave a Comment