Have you ever wondered what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6? Or what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3? Have you ever thought about what is the element of 1s 2s 2p 3s 3p? If your answer is yes, then you’re in the right place. In this blog post, we’ll take a closer look at the different elements that correspond to the different configurations of electrons.
The elements that correspond to configurations of electrons can be determined by looking at the number of protons and electrons that are present. For instance, the element with 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is Iridium, which has an atomic number of 77 and an atomic weight of 192.2. It is also a transition element, meaning that it is capable of forming bonds with other elements.
Similarly, the element with 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is Tantalum, with an atomic number of 73 and an atomic weight of 180.9. It is also a transition element, so it is capable of forming bonds. The element with 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is Rhodium, with an atomic number of 46 and an atomic weight of 102.9. It is also a transition element.
The element with 1s 2s 2p 3s 3p is Lithium, with an atomic number of 3 and an atomic weight of 6.94. It is a member of the alkali metal family, which is composed of elements that are reactive and have low melting points. The element with 1s2 2s2 2p6 3s2 3p6 is Carbon, with an atomic number of 6 and an atomic weight of 12.01. It is a non-metal element that is essential for life.
Finally, the element with 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is Nickel, with an atomic number of 28 and an atomic weight of 58.69. It is a transition element that is used in many different industries, such as electronics and jewelry-making.
As you can see, different configurations of electrons can lead to different elements. It is important to understand these different elements and their properties so that you can make informed decisions when it comes to using them in materials. Whether you’re looking for a transition element or a non-metal element, understanding the elements and their properties is key to making the right decisions.
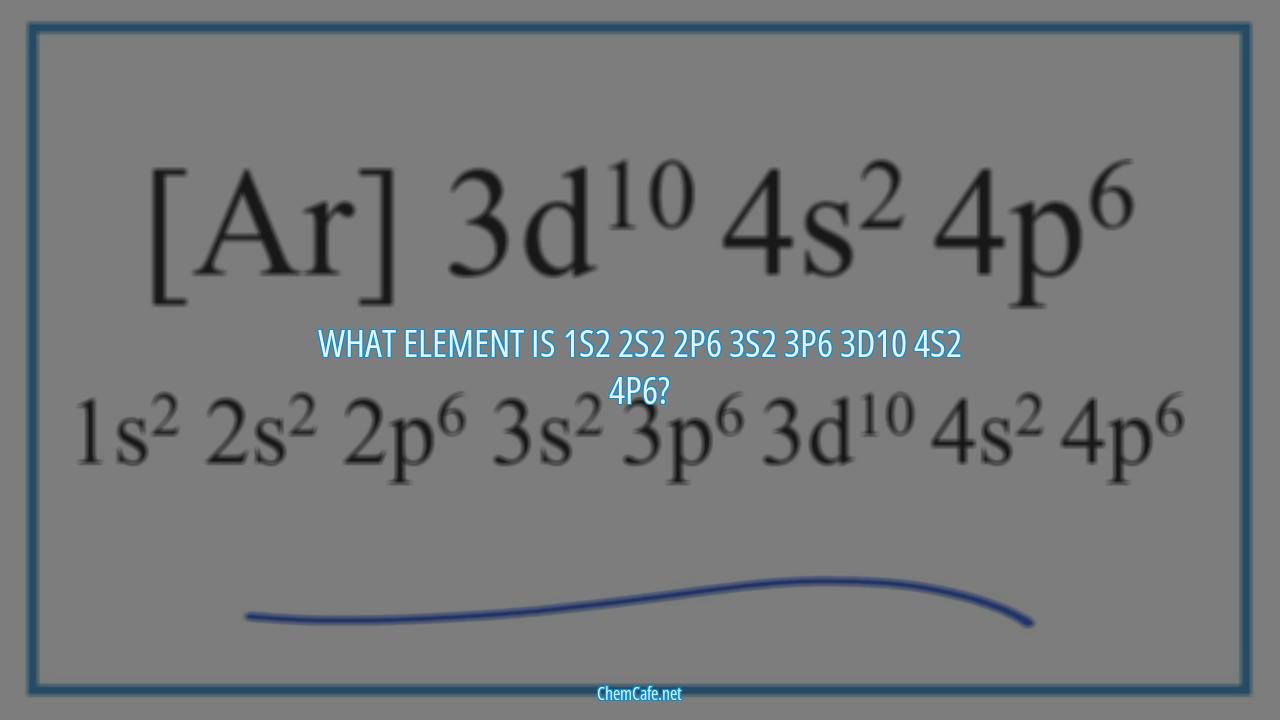
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
Are you a chemistry enthusiast looking for the answer to the question “What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?”? If so, you’ve come to the right place. In this article, we’ll explain the answer to this question, as well as provide insights into the element’s atomic weight, atomic number, group, and electron configuration.
Iridium
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is iridium. Iridium is a transition element, meaning it is in the group of elements that are found between the s- and p-blocks on the periodic table. Its atomic weight is 192.2 and its atomic number is 77. The complete electron configuration for iridium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Tantalum
Another element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is tantalum. Tantalum is also a transition element and has an atomic weight of 180.9 and an atomic number of 73. The full electron configuration for tantalum is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Rhodium
The element rhodium also has an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. Rhodium is a transition element with an atomic weight of 102.9 and an atomic number of 46. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Palladium
The final element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is palladium. Palladium is a transition element with an atomic weight of 106.4 and an atomic number of 47. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
The answer to the question “What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?” is iridium, tantalum, rhodium, and palladium. All four of these elements are transition elements and have similar atomic weights and atomic numbers. Each element also has its own unique electron configuration, which is important for understanding their properties and characteristics.
If you have any further questions about any of these elements or their electron configurations, please don’t hesitate to contact us. We’re here to help you find the answers you need.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
Atomic number, atomic weight, and electron configuration are all important elements of the periodic table. But, what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3? This electron configuration can be used to identify four elements: tantalum, iridium, rhodium, and palladium. All four elements are transition metals, and they all have similar properties. In this article, we’ll take a closer look at each of these elements to see what makes them unique.
Tantalum (Ta)
Tantalum is a transition metal with an atomic number of 73 and an atomic weight of 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3. It is a hard, silvery-gray metal that is highly resistant to corrosion and has a high melting point. In nature, it is usually found combined with other elements, and it is used in a wide range of applications, including electronics and medical implants.
Iridium (Ir)
Iridium is a transition metal with an atomic number of 77 and an atomic weight of 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. It is a very hard, brittle metal that is highly resistant to corrosion and has a high melting point. In nature, it is usually found as a trace element in meteorites, and it is used in a wide range of applications, including jewelry and medical implants.
Rhodium (Rh)
Rhodium is a transition metal with an atomic number of 46 and an atomic weight of 102.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7. It is a hard, silvery-white metal that is highly resistant to corrosion and has a high melting point. In nature, it is usually found as a trace element in platinum ore, and it is used in a wide range of applications, including jewelry and catalytic converters.
Palladium (Pd)
Palladium is a transition metal with an atomic number of 47 and an atomic weight of 106.4. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8. It is a soft, silvery-white metal that is highly resistant to corrosion and has a high melting point. In nature, it is usually found as a trace element in platinum ore, and it is used in a wide range of applications, including electronics and catalytic converters.
So, there you have it: the four elements that can be identified with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 are tantalum, iridium, rhodium, and palladium. All four elements are transition metals, and they all have similar properties. They are all highly resistant to corrosion and have a high melting point. In addition, they are all used in a wide range of applications, from jewelry and electronics to medical implants and catalytic converters.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
If you’re asking yourself what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10, you’re probably looking for the electron configuration of a specific element. This particular electron configuration is the electron configuration of three different elements: tantalum, iridium, and radium.
Tantalum
Tantalum is a transition metal that is represented by the atomic symbol Ta. It has an atomic number of 73 and an atomic weight of 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Tantalum is a hard, strong, and corrosion-resistant metal that is ductile and easily worked. It is used in a variety of applications, such as in electronics, medical devices, and aerospace components. It is also used as a substitute for platinum in jewelry.
Iridium
Iridium is a transition metal that is represented by the atomic symbol Ir. It has an atomic number of 77 and an atomic weight of 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Iridium is one of the densest and hardest metals on earth. It is extremely corrosion-resistant and can withstand extremely high temperatures. It is used in spark plugs, electrical contacts, and crucibles. It is also used as a coating on other metals to make them more durable.
Radium
Radium is an alkaline earth metal that is represented by the atomic symbol Ra. It has an atomic number of 88 and an atomic weight of 226.0. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2.
Radium is a radioactive element that is used in medical treatments, such as radiation therapy. It is also used in certain types of luminous paints and in nuclear reactors. Radium is highly toxic and must be handled with extreme caution.
So, when you’re asking yourself what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10, you’re looking for the electron configuration of tantalum, iridium, or radium. Each of these elements has its own unique properties and uses, making them valuable to many industries.
What is the element of 1s 2s 2p 3s 3p?
Element 1s 2s 2p 3s 3p is an elemental configuration of electrons in an atom. This configuration is used to describe the arrangement of electrons in an atom, including the number of electrons in each orbital and the energy level of each orbital. It is also used to explain the chemical properties of elements.
The 1s 2s 2p 3s 3p configuration is made up of the first three main energy levels, or shells. The first shell, 1s, is the closest to the nucleus and holds two electrons. The second shell, 2s 2p, holds eight electrons, and the third shell, 3s 3p, holds eighteen electrons.
The 1s Subshell
The 1s subshell is the closest to the nucleus and contains two electrons. These electrons are in the same energy level, so they both have the same energy. The 1s subshell is filled first, and its electrons are the most tightly bound to the nucleus.
The 2s and 2p Subshells
The 2s and 2p subshells are the second energy level and contain eight electrons. The 2s subshell holds two electrons and the 2p subshell holds six electrons. The 2s and 2p subshells have different energies, so the electrons in the 2s subshell are more tightly bound to the nucleus than the electrons in the 2p subshell.
The 3s and 3p Subshells
The 3s and 3p subshells are the third energy level and contain eighteen electrons. The 3s subshell holds two electrons and the 3p subshell holds six electrons. The 3s and 3p subshells also have different energies, so the electrons in the 3s subshell are more tightly bound to the nucleus than the electrons in the 3p subshell.
The Element of 1s 2s 2p 3s 3p
The element of 1s 2s 2p 3s 3p is a neutral atom that has seven valence electrons. This means that A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). Answer: 2s22p5. It has 2 + 5 = 7 valence electrons.
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Answer: 3s2. Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Answer: 5s1.
This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell. The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell.
Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5.
The element of 1s 2s 2p 3s 3p is important in understanding the structure and properties of atoms and molecules. It is also used to explain many of the chemical properties of elements, such as their reactivity and bonds. Knowing the electron configuration of an element can help us to understand its reactivity and other properties.
What element is 1s2 2s2 2p6 3s2 3p6?
Have you ever wondered what element is represented by the electronic configuration of 1s2 2s2 2p6 3s2 3p6? The answer is titanium (Ti), an elemental transition metal that belongs to group 4 of the periodic table.
Titanium is an important element with many uses in industry and science. It’s one of the strongest and lightest metals, and is used to make aircraft frames and other structural components, as well as medical implants and prosthetics. In addition, it’s a versatile material that can be used to create a wide range of products from jewelry to golf clubs.
Exploring the Electronic Configuration of Titanium
The electronic configuration of titanium is 1s2 2s2 2p6 3s2 3p6 3d2 4s2. This means that titanium has two electrons in its 1s orbital, two electrons in its 2s orbital, six electrons in its 2p orbital, two electrons in its 3s orbital, six electrons in its 3p orbital, two electrons in its 3d orbital, and two electrons in its 4s orbital.
The number of electrons in each orbital is important, as it helps us understand how titanium behaves chemically. Titanium has a total of fourteen electrons, which means that it is an atom with an incomplete outer shell of electrons. This means that titanium is likely to form bonds with other elements in order to fill its outer shell and become more stable.
Titanium’s Place in the Periodic Table
Titanium belongs to group 4 of the periodic table, which includes other transition metals such as vanadium, chromium, and manganese. Transition metals are characterized by their ability to form multiple bonds, and titanium is no exception. It is able to form strong covalent bonds with other elements, as well as weaker ionic bonds.
Titanium is also a member of period 5 of the periodic table, which also includes the elements scandium, yttrium, and lutetium. These elements all have similar properties, such as high melting and boiling points, and the ability to form multiple bonds.
Titanium’s Role in Industry and Science
Titanium is an important element in industry and science. It’s used to make aircraft frames and other structural components, as well as medical implants and prosthetics. It’s also used to make jewelry and golf clubs.
In addition, titanium is used in many scientific applications, such as nuclear reactors and as a catalyst in chemical reactions. It’s also used as a pigment in paints and as an additive in some foods.
Conclusion
Titanium is an important element with many uses in industry and science. It has the electronic configuration of 1s2 2s2 2p6 3s2 3p6 3d2 4s2, making it part of group 4 of the periodic table and period 5. Titanium is able to form strong covalent bonds with other elements, as well as weaker ionic bonds, and is used in many industrial and scientific applications.
What is this element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
An Overview
Have you ever wondered what an element looks like on the periodic table? Do you know what an element’s electron configuration is? Understanding the electron configuration of an element is essential in understanding its properties and behavior. In this blog post, we will explore the electron configuration of the element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, and discuss its implications.
What is the Electron Configuration?
The electron configuration of an element is the arrangement of electrons in its atomic orbitals. The number of electrons in an element’s outermost orbit is referred to as its valence electrons, and these are the electrons that are involved in chemical reactions. The electron configuration of an element is written in the form of a series of numbers and letters, with each number and letter representing the number of electrons in each orbital.
What Does 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 Mean?
The element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is a transition element with an atomic number of 46 and an atomic weight of 102.9. This means that it has 46 protons and 46 electrons. The electron configuration of this element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, which means that it has two valence electrons in the 1s orbital, two valence electrons in the 2s orbital, six valence electrons in the 2p orbital, two valence electrons in the 3s orbital, six valence electrons in the 3p orbital, two valence electrons in the 4s orbital, ten valence electrons in the 3d orbital, and three valence electrons in the 4p orbital. This element is named rhodium and belongs to the transition element group.
What Are the Implications of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 has several implications. First, it tells us that this element has a total of 46 electrons, which is consistent with its atomic number. Second, it tells us that this element has two valence electrons in the 1s orbital, two valence electrons in the 2s orbital, six valence electrons in the 2p orbital, two valence electrons in the 3s orbital, six valence electrons in the 3p orbital, two valence electrons in the 4s orbital, ten valence electrons in the 3d orbital, and three valence electrons in the 4p orbital. This means that it has a total of seven valence electrons, which is consistent with its position on the periodic table. It also tells us that this element is a transition element, which is important because transition elements have unique chemical properties that set them apart from other elements.
In conclusion, the electron configuration of the element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 tells us that it has 46 electrons, seven valence electrons, and that it is a transition element. Understanding the electron configuration of an element can help us to better understand its chemical properties and behavior.



Leave a Comment