Atoms are the building blocks of everything in the universe, from the massive stars in our galaxy to the very cells that make up our bodies. They are composed of protons, neutrons, and electrons, and the number of each determines the element they make up. In this article, we will explore the atomic structure of elements with 15 electrons and 17 neutrons and their isotopes.
Atoms are composed of protons, neutrons, and electrons. The number of protons determines the element, while the sum of protons and neutrons gives the atomic mass. Isotopes are identified by their mass, which is the total number of protons and neutrons. Each element has multiple isotopes, with slightly different numbers of neutrons, such as phosphorus, which has 16 neutrons, and chlorine, which has 17 neutrons.
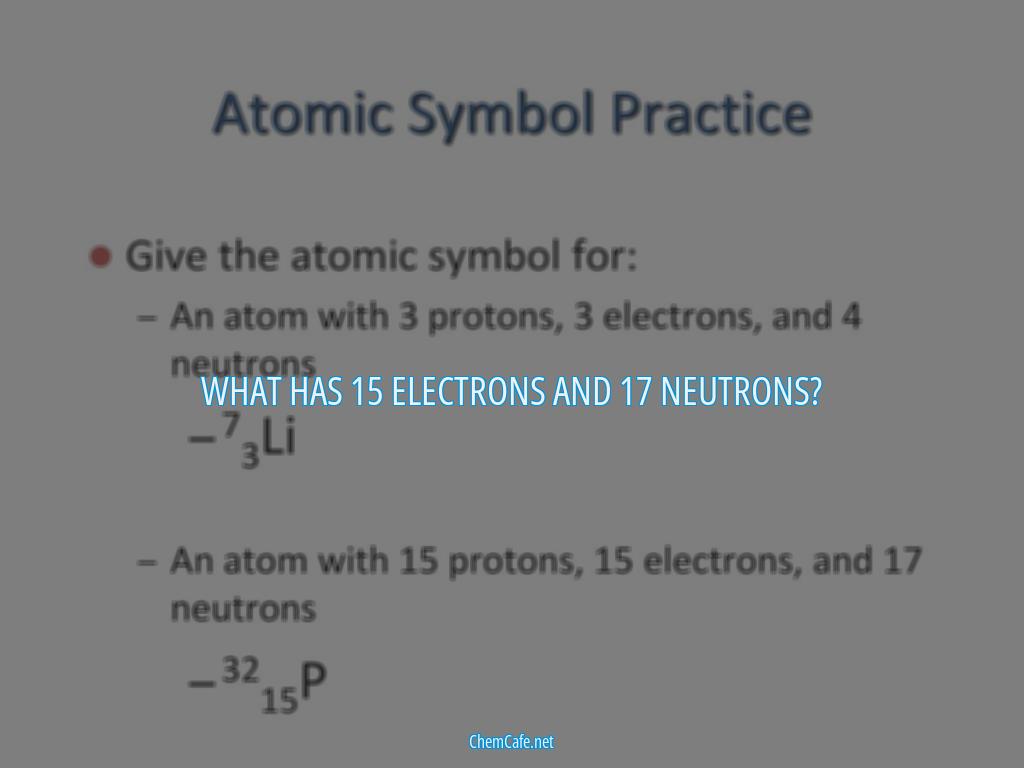
The element with 15 protons and 17 neutrons is phosphorus. Phosphorus is a non-metal and is essential for life on Earth, as it is found in proteins, cell membranes, and DNA. It is also a component of many fertilizers and is used in matches, fireworks, and explosives. Phosphorus has 16 neutrons, making it a stable isotope.
When an atom has 15 protons and 15 electrons, it is carbon-15. Carbon-15 is a radioactive isotope of carbon. It is used in research to trace biochemical reactions and determine the age of fossils. Carbon-15 has a half-life of about 5,370 years, meaning that after 5,370 years, half of the atoms will have decayed.
When an element has 15 protons and 16 neutrons, it is sulfur-30. Sulfur-30 is a stable isotope of sulfur and is found in rocks, soil, and water. It is also used in medical imaging, as it emits gamma rays when exposed to radiation.
Finally, when an element has 15 protons and 17 neutrons, it is chlorine-35. Chlorine-35 is a stable isotope of chlorine and is found in seawater and salt deposits. It is also used in medical and industrial imaging and has a half-life of about 37 minutes.
In summary, atoms are composed of protons, neutrons, and electrons, and the number of each determines the element. Atoms with 15 protons and 17 neutrons are chlorine-35, which is a stable isotope of chlorine. Other isotopes with 15 protons and 16 or 15 neutrons are phosphorus and carbon-15, respectively. Understanding the atomic structure of elements can help us to better understand their properties and uses.
What has 15 electrons and 17 neutrons?
An Overview
Atoms are made up of protons, neutrons, and electrons. The number of protons and electrons is equal to the atomic number Z = 15. The mass number is the sum of atomic number and neutrons that is A = 15 + 17 = 32. This element is phosphorus.
Isotopes are atoms with the same atomic number but different mass numbers, meaning they have different numbers of neutrons. All isotopes of an element have the same number of protons but different number of neutrons. This means that an atom with 15 electrons, 17 neutrons, and 15 protons is an isotope of phosphorus.
What are Stable Isotopes?
The nucleus of each atom contains protons and neutrons. While the number of protons defines the element (e.g., hydrogen, carbon, etc.) and the sum of the protons and neutrons gives the atomic mass, the number of neutrons defines the isotope of that element. For example, most carbon (≈ 99%) has 6 protons and 6 neutrons and is written as 12C to reflect its atomic mass.
Stable isotopes are atoms with the same atomic number and mass number. They are not radioactive, meaning they do not undergo radioactive decay. Stable isotopes are important for a number of scientific and industrial applications, including medical imaging, nuclear power, and carbon dating.
Why is the 15-17 Isotope of Phosphorus Important?
The 15-17 isotope of phosphorus is the most common isotope of phosphorus found in nature. It is found in biological systems, including plants and animals. It is also used in a variety of industrial and research applications.
In medical imaging, the 15-17 isotope of phosphorus is used to detect tumors, measure blood flow, and assess the effectiveness of treatments. It is also used in nuclear power plants to measure the amount of energy released during a nuclear reaction. In carbon dating, it is used to determine the age of materials that contain carbon.
Atoms with 15 electrons, 17 neutrons, and 15 protons are an isotope of phosphorus. This isotope is stable, meaning it does not undergo radioactive decay. It is the most common isotope of phosphorus found in nature and is used for a variety of scientific and industrial applications, including medical imaging, nuclear power, and carbon dating.
What has 16 neutrons and 15 electrons?
Atoms are the smallest particles of matter and are composed of protons, neutrons and electrons. The number of protons, neutrons and electrons determine the type of element an atom is. One particular atom has 15 protons, 16 neutrons, and 15 electrons, and its charge is zero. Understanding the structure of this atom can help us understand the behavior of atoms in general.
The Nucleus
At the center of each atom is the nucleus, which contains the protons and neutrons. The number of protons in an atom determines what element it is. Therefore, this atom is an element with 15 protons, and is therefore a phosphorus atom. Protons have a positive charge, while neutrons have no charge. The nucleus also contains neutrons, which help to stabilize the atom. In this particular atom, there are 16 neutrons.
The Electrons
The electrons are located outside of the nucleus, and are the particles responsible for the chemical behavior of the atom. Electrons have a negative charge, and in this particular atom, there are 15 electrons. The electrons are arranged in specific shapes, called orbitals, around the nucleus. The number of electrons in each orbital depends on the element, and for this atom, each orbital contains two electrons.
The Charge
Atoms are neutral, meaning they have no charge. The number of protons and electrons in an atom must be equal in order for the atom to be neutral. In this atom, there are 15 protons and 15 electrons, so the charge is zero.
Isotopes
Isotopes are atoms of the same element that have a different number of neutrons. Since the number of protons determines the type of element, isotopes of different elements can have the same number of protons and electrons. This particular atom is an isotope of phosphorus, since it has 15 protons and 16 neutrons.
In conclusion, this atom has 15 protons, 16 neutrons, and 15 electrons. The number of protons determines the type of element, and in this case, the element is phosphorus. The number of neutrons determines the isotope of the element, and in this case, the isotope is phosphorus-16. The number of electrons and protons must be equal in order for the atom to be neutral, and in this case, the charge of the atom is zero.
Why does phosphorus have 16 neutrons?
Phosphorus is an essential element for life, and it is important to understand the atomic structure of phosphorus to understand why it has 16 neutrons. The number of neutrons in an atom is determined by the mass number and the atomic number of that element. Phosphorus has an atomic number of 15, meaning it has 15 protons, and a mass number of 31, meaning its total number of protons and neutrons is 31. Therefore, the number of neutrons in phosphorus is 16, which is 31 minus the 15 protons.
Phosphorus is an element found in the middle of the periodic table, and its atomic number is 15. This number corresponds to the number of protons in phosphorus, and it is also equal to the number of electrons. The mass number of phosphorus is 31, and this number is the sum of the protons and neutrons in phosphorus. To calculate the number of neutrons in phosphorus, one simply needs to subtract the number of protons (15) from the mass number (31). This gives us 16 neutrons in phosphorus.
How is the number of neutrons in an atom determined?
The number of neutrons in an atom is determined by its mass number and atomic number. The atomic number of an element is equal to the number of protons in the atom, and it is also equal to the number of electrons. The mass number is equal to the sum of the protons and neutrons in an atom. To calculate the number of neutrons in an atom, one simply needs to subtract the number of protons from the mass number.
What is the importance of phosphorus?
Phosphorus is an essential element for life, and it plays an important role in many biological processes. It is involved in the formation of bones, teeth, and other tissues, and it is also essential for the production of energy in cells. Phosphorus is also essential for the formation of DNA, and it is essential for the formation of many proteins and enzymes in the body. Phosphorus is an essential nutrient for plants and animals, and it is a key component of many fertilizers.
What is β decay?
β decay is a form of radioactive decay in which an unstable nucleus emits an electron (β particle) and transforms into a daughter nucleus with a higher atomic number. In β decay, one neutron decays to one proton with the emission of a beta particle. This means that in the daughter nucleus the number of protons increases by one and the number of neutrons decreases by one.
In summary, phosphorus has an atomic number of 15 and a mass number of 31. This means that there are 15 protons and 16 neutrons in phosphorus. The number of neutrons in an atom is determined by its mass number and atomic number, and it is calculated by subtracting the number of protons from the mass number. Phosphorus is an essential element for life, and it is involved in many important biological processes. β decay is a form of radioactive decay in which an unstable nucleus emits an electron and transforms into a daughter nucleus with a higher atomic number.
What is an atom with 15 protons and 15 electrons?
Atoms are the building blocks of matter, and all matter is composed of atoms. They are the smallest particle of an element that retains the chemical properties of that element. Atoms are made up of three subatomic particles: protons, neutrons, and electrons. The number and arrangement of these particles determine the element’s chemical properties.
The number of protons available in an atom determines what element it is. Each element has a unique number of protons, and this number is known as the atomic number. The number of protons, neutrons, and electrons in an atom are collectively called its atomic mass.
An atom with 15 protons and 15 electrons has an atomic number of 15 and an atomic mass of 30. This element is phosphorus, and it has 16 neutrons. The total number of subatomic particles in this atom is 32.
Charge of an Atom with 15 Protons and 15 Electrons
The charge of an atom is determined by the number of protons and electrons it has. Since this atom has 15 protons and 15 electrons, it has a neutral charge. This means that the number of protons and electrons cancel each other out and the atom has no overall charge.
What Happens When an Atom Loses or Gains Electrons?
Atoms can gain or lose electrons, which affects their charge. When an atom gains an electron, it becomes negatively charged as the number of electrons is greater than the number of protons. When an atom loses an electron, it becomes positively charged as the number of protons is greater than the number of electrons.
Radioactive Decay
Atoms can also undergo radioactive decay, which is when an atom spontaneously changes into a different element by releasing energy in the form of radiation. In this process, an atom can lose or gain protons, neutrons, or electrons.
For example, a phosphorus atom with 15 protons and 15 electrons can undergo radioactive decay and change into a sulphur atom. In this process, the phosphorus atom loses one electron and gains one proton, resulting in 16 protons and 16 electrons. This changes the atomic number from 15 to 16 and the atomic mass from 30 to 32.
An atom with 15 protons and 15 electrons has an atomic number of 15 and an atomic mass of 30. This element is phosphorus, and it has 16 neutrons. The charge of this atom is neutral as the number of protons and electrons cancel each other out. Atoms can gain or lose electrons and protons, which affects their charge. They can also undergo radioactive decay, which is when an atom spontaneously changes into a different element by releasing energy in the form of radiation.
What element has 15 protons 16 neutrons and 15 electrons?
Atoms are the building blocks of matter, and they are made up of three main particles: protons, neutrons, and electrons. The number of protons available in an atom determines its identity, making it a unique element. But what element has 15 protons, 16 neutrons, and 15 electrons?
The answer is Phosphorus (P). Phosphorus is one of the 118 elements in the periodic table and is represented by the symbol P. It has an atomic number of 15 and a mass number of 31. This means that it has 15 protons and 16 neutrons, and the number of electrons is equal to the number of protons, making it 15.
What is an Atom?
Atoms are the smallest particles of matter that make up all living and non-living things. They are made up of three main particles: protons, neutrons, and electrons. Protons are positively charged particles located in the nucleus of the atom, while electrons are negatively charged particles found in the electron cloud around the nucleus. And neutrons are the neutrally charged particles also found in the nucleus of the atom. The nucleus is the region at the center of the atom that contains the protons and neutrons, whereas the atom is the entire structure of protons, neutrons, and electrons arranged in a nucleus and electron cloud.
How to Identify an Element?
The number of protons available in an atom determines its identity, making it a unique element. We can use this information to identify any element by using its atomic number and mass number. The atomic number of an element is equal to the number of protons, and the mass number is the sum of the protons and neutrons in the nucleus.
Calculating the Number of Protons, Neutrons, and Electrons
Given the number of protons and neutrons, we can calculate the number of electrons. We can also use the atomic number and mass number to calculate the number of protons and neutrons.
Number of Electrons
The number of electrons in an atom is equal to the number of protons. This means that if an atom has 15 protons, it will also have 15 electrons.
Number of Protons and Neutrons
To calculate the number of protons and neutrons, we can use the formula:
Atomic number=Number of protons=Number of electrons
Mass number=Number of protons+Number of neutrons
Given: Number of protons=15
Number of neutrons=16
Calculate the atomic number of the element:
Atomic number=Number of protons
Thus,
Atomic number=15
Thus, the atomic number of the element is 15.
Calculate the mass number of the element:
Mass number=Number of protons+Number of neutrons
Thus,
Mass number=15+16
Mass number=31
Thus, the mass number of the element is 31.
Represent the Element
The element with atomic number 15 is Phosphorous (P). The general representation of elements is done as XZA. Substitute P for the chemical symbol, 15 for the atomic number and 31 for the mass number.
Thus, the representation of element is P1531.
So, to answer the question, the element with 15 protons, 16 neutrons, and 15 electrons is Phosphorus. Phosphorus is represented by the symbol P and has an atomic number of 15 and a mass number of 31.
What element has 15 protons and 17 neutrons and 15 electrons?
Atoms are made up of protons, neutrons, and electrons. The number of protons in an atom determines its identity, while the number of neutrons and electrons can vary. This means that there are different isotopes of the same element, which have the same number of protons but different numbers of neutrons and electrons.
The Basics of Isotopes and Atomic Structure
Atoms are composed of three particles: protons, neutrons, and electrons. The number of protons in an atom determines the element it is, while the number of neutrons and electrons can vary. The sum of the protons and the neutrons is called the atomic mass or molecular weight.
The difference in the number of neutrons in atoms of the same element is what makes them isotopes. An isotope is an atom of the same element but with a different number of neutrons. Isotopes have different physical and chemical properties, and can be used for various applications, such as medical imaging, radiotherapy, and carbon dating.
The Isotope with 15 Protons, 17 Neutrons, and 15 Electrons
Atoms with 15 protons and 15 neutrons are expected to have 15 protons, 15 electrons, and more or less than 15 neutrons. In this case, the atom has 17 neutrons and 15 electrons, so it is an isotope of the element with 15 protons.
The isotope of an atom with 15 protons and 17 neutrons is expected to have 15 protons, 17 neutrons, and 15 electrons. This isotope is known as Carbon-15 or C-15. It is a naturally-occurring isotope of carbon, and it is the most abundant isotope of carbon on Earth.
Uses of Carbon-15
Carbon-15 has a variety of applications. It is used in medical imaging, radiotherapy, and carbon dating. In medical imaging, C-15 is used to detect cancer and other diseases, as well as to monitor the progress of treatments. In radiotherapy, it is used to treat cancer with radiation. And in carbon dating, it is used to determine the age of fossils and artifacts.
The isotope of an atom with 15 protons and 17 neutrons is expected to have 15 protons, 17 neutrons, and 15 electrons. This isotope is known as Carbon-15 or C-15. It is a naturally-occurring isotope of carbon and is used in medical imaging, radiotherapy, and carbon dating.




Leave a Comment