What Is an Orbital?
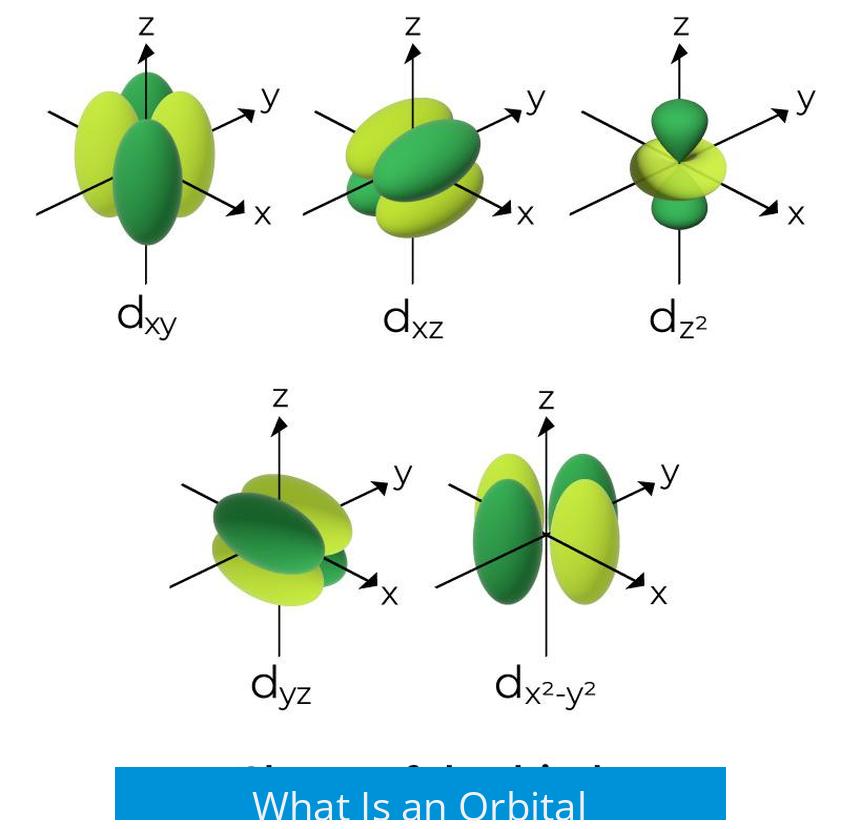
An orbital is a mathematical description of a region around an atomic nucleus where there is a high probability of finding an electron, typically expressed by solutions to the Schrödinger equation that specify electron behavior in terms of energy and spatial distribution.
Electron Waves and the Origin of Orbitals
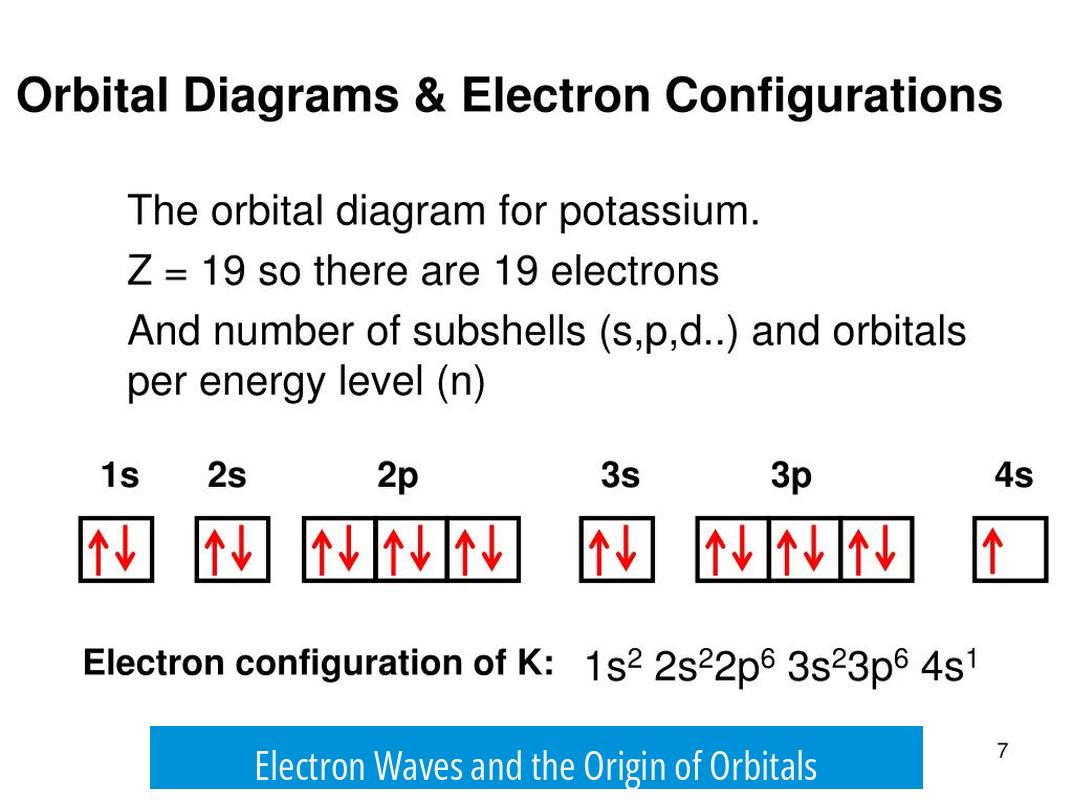
Electrons exhibit wave-particle duality, behaving both as particles and waves. The wave aspect is key for understanding orbitals. Instead of viewing electrons as discrete points, they are considered as wavefunctions, which describe the probability distribution of their location around an atom.
When electrons are bound to atoms, the waves form standing wave patterns. These standing waves have specific energies corresponding to allowed quantum states. The shape and energy of these standing waves correspond to what we call orbitals.
Quantum Numbers and Orbital Shapes
Orbitals are described using quantum numbers representing different properties:
- Principal quantum number (n): relates to the overall size and energy of the orbital and roughly counts nodes in the wavefunction.
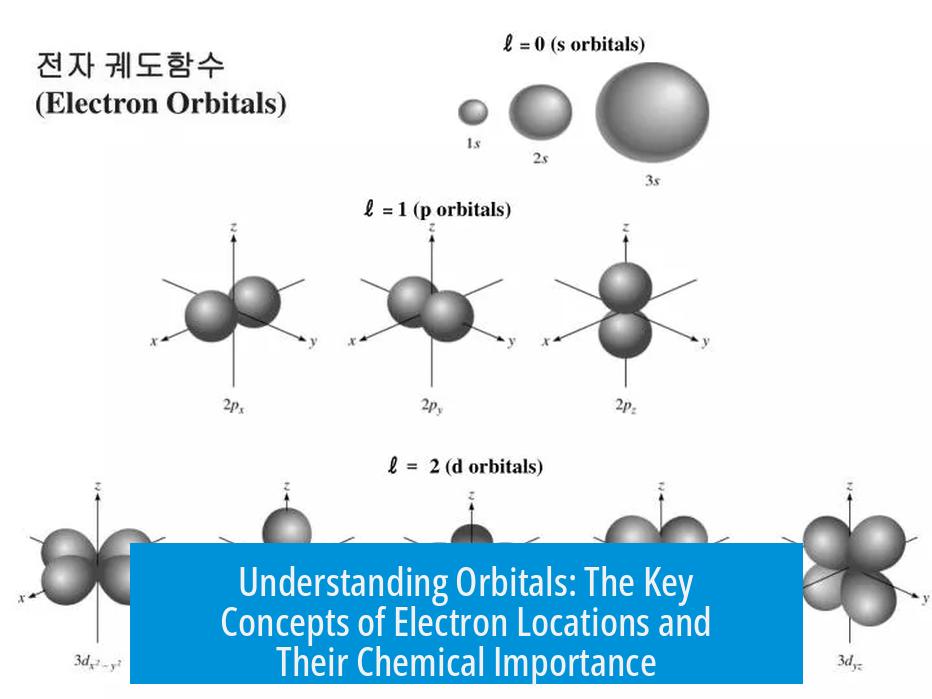
- Azimuthal quantum number (l): describes the shape and number of non-spherical nodes in the orbital; for example, spherical for s orbitals (l=0), dumbbell-shaped for p orbitals (l=1), and more complex shapes for d (l=2) and f (l=3) orbitals.
- Magnetic quantum number (ml): specifies the orientation in space of the orbital’s shape.
Nodes are regions where the probability of finding an electron is zero. In three dimensions, these nodes form surfaces that define the intricate shapes of orbitals.
Shapes and Types of Atomic Orbitals
| Orbital Type | Shape | Nodes | Example |
|---|---|---|---|
| s | Spherical | Only spherical nodes (concentric shells) | 1s, 2s |
| p | Dumbbell-shaped | One planar node dividing lobes | 2p, 3p |
| d | More complex (cloverleaf or donut shapes) | Two planar or conical nodes | 3d, 4d |
| f | Even more complex shapes | Three or more nodes | 4f, 5f |
These orbital shapes reflect areas where electrons can be found with high probability but are not hard boundaries. Real orbitals can distort based on the presence of other atoms or electric fields.
Orbital Capacity and Chemical Significance
Each orbital can contain a maximum of two electrons. This limitation arises from electron spin, where two electrons in the same orbital must have opposite spins.
Orbitals are fundamental in understanding chemical bonding. Electrons in orbitals participate in forming molecules by overlapping with orbitals from other atoms. Molecular orbitals form from linear combinations of atomic orbitals. For instance, the hydrogen molecule (H2) forms when two 1s atomic orbitals overlap, sharing electrons between nuclei.
The Orbital Model: A Conceptual Tool
Orbitals are not physical objects but mathematical models that describe where electrons probably exist. They emerge from solving the Schrödinger equation for electrons in atoms or molecules.
In organic chemistry, orbitals underlie reactivity patterns. The shape, size, and energy of an orbital explain how atoms interact, how bonds form or break, and the spatial arrangement of molecules.
Advanced Considerations
At more advanced levels, molecular orbitals explain properties such as oxidation states, ligand behavior in coordination complexes, and electronic symmetry. The orbital concept extends beyond isolated atoms to complex molecules and materials.
Key Takeaways
- An orbital is a three-dimensional region where there is a significant probability of finding an electron.
- Orbitals arise from electron wave behavior described by the Schrödinger equation.
- Quantum numbers n, l, and ml define the energy, shape, and orientation of orbitals.
- Common orbital types are s (spherical), p (dumbbell), d, and f, each with characteristic shapes.
- Each orbital holds up to two electrons with opposite spins.
- Orbitals help explain chemical bonding as regions where electrons are shared or transferred.
- Orbitals are conceptual rather than physical entities, useful models for electron behavior.
What Is an Orbital? Unlocking the Mystery of Electron Homes
Simply put, an orbital is a three-dimensional region around an atom’s nucleus where there is a very high chance—usually about 90 to 95 percent—of finding an electron. But wait! That definition barely scratches the surface of this fascinating quantum doodad. Let’s deep dive into the quirky world of orbitals, quantum mechanics, and why electrons are always throwing wavy dance parties in invisible spaces.
So, what exactly is an orbital? At first glance, you might picture electrons zipping around the nucleus in neat little orbits like tiny planets. Sorry, but that mental image belongs in the 1800s. Modern science reveals a moody electron behaves less like a pinpoint particle and more like a waving, probability-spinning cloud. Welcome to the wave-particle duality!
Electrons: Both Particles and Waves. Say What?
First, brace yourself. Electrons are quantum shape-shifters. According to quantum mechanics, they have a dual personality—they can act like discrete particles and like waves, often simultaneously. This duality means that the electron isn’t just buzzing in a tiny circle but instead inhabits a complex wavelike existence.
Now focus on the wave side of this story. These electron waves aren’t your typical ocean waves or guitar strings. Electron waves describe the probability distribution—the likelihood of finding the electron in a certain region around the nucleus. The “height” of the wave corresponds to the square root of probability density. It’s abstract, sure, and guess what? Imaginary numbers play a starring role here (yes, imaginary, as in involving the square root of negative one). If that sounds like an episode of a sci-fi series, you’re on the right track.
Standing Waves and Orbitals: The Quantum Dance Floors
Here’s a nifty concept: when electrons are bound to an atom, they form “standing waves,” somewhat like the vibrations of a plucked string on a guitar, but in three dimensions and much, much smaller.
Imagine plucking a string and seeing vibrating nodes—points that don’t move. For electrons, these nodes become surfaces or shapes in 3D space where the wave amplitude is zero. The number of nodes relates directly to the energy of the electron’s wave; more nodes mean higher energy levels. These standing wave patterns are what define orbitals! In other words, orbitals are the quantum music notes corresponding to the allowed energy patterns of electrons.
The Three Dimensions of Electron Waves: More Than Just a String
It’s tempting to compare these waves to vibrations on a string, but electrons wiggle in three-dimensional space. Imagine the whole sphere buzzing, with nodes as surfaces, not just dots or lines. The shapes formed are fascinating: spherical, dumbbell-shaped, cloverleaf patterns, and more. These shapes help chemists visualize where it’s “most likely” to find an electron.
Quantum Numbers: The Orbital Coordinates
To track these orbitals, we turn to quantum numbers, kind of like a GPS for electron waves.
- n: The principal quantum number counts the total number of nodes plus one and roughly corresponds to the energy level or “shell” of an electron.
- l: The angular momentum quantum number tells us the shape of the orbital — s, p, d, or f. Think of it as the “model” of the stage where electrons perform.
- ml: The magnetic quantum number specifies the orientation of the orbital in space.
For example, s orbitals (where l=0) are perfectly spherical. In contrast, p orbitals (l=1) look like dumbbells with one planar node slicing through the nucleus, defining regions of zero probability.
Orbitals Are Not Perfect: Real Electrons Play Dirty
Even though we get these neat shapes, real-world orbitals aren’t perfect spheres or dumbbells frozen in space. The nucleus’s pull and the presence of other electrons distort these idealized shapes. Imagine trying to balance a wobbling, irregularly shaped jelly—same deal in quantum land!
Orbitals: The Building Blocks of Chemistry
Why care about orbitals? Because electrons are the workhorses of chemistry, dictating how atoms bond, react, and form the tapestry of matter. You can think of orbitals as distinct “homes” where electrons live. The size, shape, and filling status of these homes predict chemical behaviors.
Chemists talk about orbitals being “unfilled,” “partially filled,” or “full,” terms critical for understanding molecule reactivity. Concepts like HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) help explain how molecules interact and react. Pretty fancy stuff for an invisible quantum house!
Orbitals—Models, Not Physical Things
Keep in mind, orbitals aren’t tangible objects; they’re mathematical constructs—solutions to the Schrödinger equation, the grand formula of quantum mechanics describing how electrons behave. They’re like blueprints of where an electron is likely to be, akin to a weather forecast. We don’t see the storm, but the radar shows where conditions favor rain.
Hybrid and Molecular Orbitals: Teamwork in Chemistry
Atomic orbitals get cooler when atoms bond. Hybrid orbitals arise when atomic orbitals combine within one atom to form new shapes—this is foundational in organic chemistry. Molecular orbitals come next—formed by overlapping atomic orbitals from different atoms, creating bonds. Hydrogen molecule (H2) is a classic example where two s orbitals combine, sharing electrons and forming a covalent bond.
The Periodic Table and Orbitals: A Quantum Map
Ever noticed the periodic table’s blocks? Those s, p, d, and f blocks directly relate to orbitals. Elements in the s-block have electrons filling s orbitals, p-block elements fill p orbitals, and so forth. Each orbital can hold a maximum of two electrons, distinguished by their spin, neatly filling shells and subshells according to quantum rules.
The Probability Cloud: More Than Just a Blurry Photo
Orbitals are often shown as fuzzy “electron clouds.” This cloud represents zones where the electron’s presence is most probable. Visualize it like a fog around a streetlamp—the denser the fog, the greater the probability. Now, when these clouds overlap between atoms, they build molecular orbitals, the invisible glue holding molecules together.
Beyond Basics: Orbitals in Advanced Inorganic Chemistry
For those who enjoy chemistry on steroids, orbitals help explain metal oxidation states, ligand effects, and molecular symmetry. By understanding the shape and energy of these orbitals, chemists can predict everything from color to magnetic properties in metal complexes. It’s the secret sauce making chemistry both predictable and delightfully weird.
Philosophical Rabbit Holes: What Is an Orbital, Really?
Dare to think deeper, and orbitals become a doorway to mind-boggling questions. They’re mathematical constructs that describe probabilities, but what does that say about the electron’s “actual” existence? Some even stumble into theories proposing all electrons might be the same particle hopping around spacetime—a quantum conspiracy! But we’ll stop before we dive into that wormhole.
Summary Table: Key Facts About Orbitals
| Aspect | Explanation |
|---|---|
| Definition | Region around the nucleus with high probability (~90-95%) of finding an electron. |
| Nature | Mathematical solutions to the Schrödinger equation; wave functions representing electron behavior. |
| Electron Capacity | Max two electrons per orbital, distinguished by opposite spins. |
| Types | s (spherical), p (dumbbell), d, f orbitals – each with unique shapes. |
| Quantum Numbers | n, l, ml describe energy level, shape, and orientation. |
| Role in Chemistry | Foundational for chemical bonding, molecular formation, and reactivity. |
Orbitals in Everyday Chemical Life
Orbitals might seem like abstruse quantum jargon, but they are ground zero for everything chemical around you. The soap that cleans, the oxygen you breathe, the DNA in your cells—all owe their existence to electrons dancing in orbitals.
When students prepare for exams, a practical working definition helps: “An orbital is a region of space around the nucleus where there is greater than 99% chance of finding an electron.” It’s simple, neat, and gets you through most questions. However, the real magic happens when you explore the shapes, hybridizations, and overlaps—essentially, the electron’s playground that unlocks molecular complexity and chemical creativity.
Want to Visualize Orbitals?
Text alone can’t capture their beauty. A few recommended sites help visualize these shapes:
- Ptable.com orbital visualizer – interactive and colorful illustrations of orbitals.
- 2D standing wave animations – helpful analogy for understanding vibrational modes.
- Standing waves on a string gif – visualize fundamental waves before scaling to 3D orbitals.
Final Thoughts: Orbitals, The Quantum Real Estate
Orbitals are not just fancy rocks on a table; they are the invisible plots of cosmic land where electrons dwell. Understanding these makes chemistry less mystifying and more like reading the blueprint of the universe. Next time you hear “orbital,” picture not a boring old circle, but a dynamic 3D cloud, a vibrating quantum dance floor, or the ultimate electron mansion.
Curious where your electrons are hanging out right now? That question alone opens doors into quantum mechanics, chemistry, and, if you’re brave, some deep philosophical musings.
As you venture further into chemistry, orbitals become trusted guides unraveling molecular mysteries and predicting chemical behavior—making the hard science surprisingly human and a bit quirky.
What exactly is an orbital in an atom?
An orbital is a region in space around the nucleus where there is a high probability of finding an electron. It is a three-dimensional standing wave pattern that results from the electron’s wave-like nature.
How do orbitals relate to electron waves?
Orbitals come from electrons behaving as waves. These electron waves form standing wave patterns when bound to atoms, and each pattern corresponds to an orbital with specific energy and shape.
What do quantum numbers tell us about orbitals?
Quantum numbers describe the shape, size, and orientation of orbitals. For example, n defines energy levels, l the number of nodal surfaces, and ml their orientation in space.
Why are orbitals important in chemistry?
Orbitals determine where electrons are likely to be. Their shapes and occupancy affect chemical bonding, reactions, and molecular structure, making them fundamental in understanding chemistry.
Are orbitals physical objects?
No, orbitals are mathematical models based on the Schrödinger equation. They describe where electrons are likely found but don’t represent physical “objects” you can see.
What is the difference between atomic and molecular orbitals?
Atomic orbitals exist around single atoms. Molecular orbitals form when atomic orbitals from different atoms combine, describing electron distribution in molecules and bonds.




Leave a Comment