Understanding the pKa of Hydroxide: Clarifying Common Misconceptions
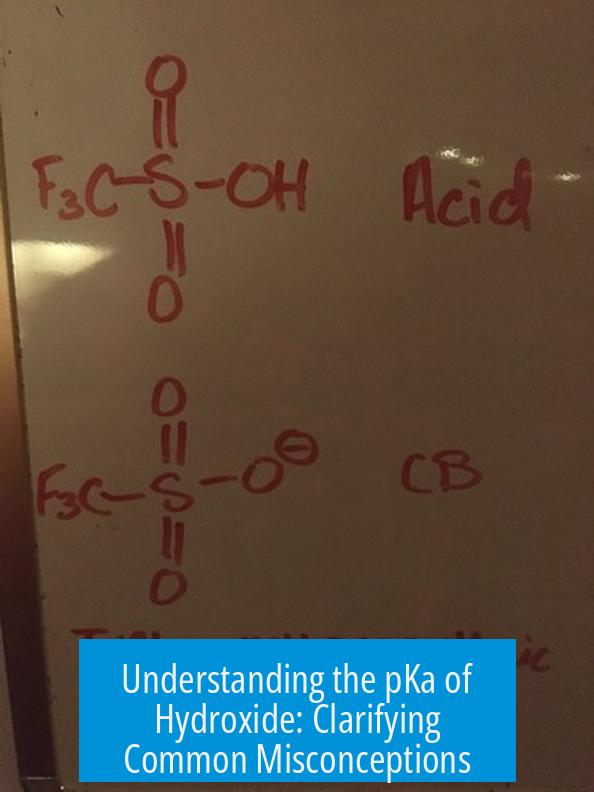
The pKa of hydroxide (HO−) itself is not a commonly referenced value because hydroxide is a base rather than an acid. Instead, pKa values typically relate to the acidity of water (H2O) converting to hydroxide (HO−), which is about 14 in aqueous solution. However, the further deprotonation of hydroxide to oxide ion (O2−) has an unmeasurably high pKa, well above 16, reflecting an extremely weak acidity and very strong basicity.
Defining pKa and its Context
pKa quantifies the acidity of a protonated species. It expresses the equilibrium constant for the loss of a proton (H+). This means pKa pertains to acids losing protons, whereas bases gain protons.
For hydroxide, discussion of pKa involves two main acid-base equilibria:
- Water (H2O) ⇌ Hydroxide (HO−) + H+ — This refers to water acting as an acid, with hydroxide as its conjugate base.
- Hydroxide (HO−) ⇌ Oxide Ion (O2−) + H+ — Deprotonation of hydroxide to form oxide ion.
pKa of Water (H2O) → Hydroxide (HO−)
The acidity of water is well established and experimentally measurable in water itself. The commonly accepted pKa for the reaction H2O ⇌ HO− + H+ is around 14 at 25°C. This reflects the autoionization constant of water (Kw ≈ 10−14).
Despite some claims suggesting a value of 15.7, the consensus supported by reliable measurements is about 14. This means water is a very weak acid but does lose protons to produce hydroxide ions at low concentrations.
The pKa of Hydroxide (HO−) → Oxide Ion (O2−)

Hydroxide can, in principle, lose an additional proton to give the oxide ion:
HO− ⇌ O2− + H+
The oxide ion (O2−) carries a -2 charge, making it a far stronger base than hydroxide (-1 charge). The conjugate acid of oxide, hydroxide, is thus a very weak acid.
In water, this second deprotonation’s pKa is so high that it is practically immeasurable. It exceeds 16, and possibly is comparable to extremely weak acids such as alkanes, whose pKa values exceed 50.
Experimental measurement is hindered for multiple reasons:
- Oxide ions are very reactive and tend to immediately react with water, making their isolation in aqueous solution impossible.
- Oxide salts are often insoluble in water, precluding direct measurement.
- Alternative solvents such as Dimethylformamide (DMF) or Dimethylsulfoxide (DMSO) might allow measurement, but these data are sparse and difficult to obtain.
Why Does Hydroxide’s Negative Charge Not Give It a Lower pKa?
It can seem counterintuitive that hydroxide, being negatively charged, is not more basic with a pKa above 16. However, pKa measures the strength of an acid, i.e., how easily its proton dissociates, not the base’s affinity for protons directly.
Hydroxide is the conjugate base of water, which has a pKa of 14. The proton attached to oxygen in hydroxide is far less acidic than in water because removing the proton results in oxide ion, which is highly unstable in aqueous solution due to its high charge density.
The presence of a negatively charged oxygen in hydroxide makes it a strong base because it readily attracts protons. However, the acidity of hydroxide itself is related to the ability to lose its proton, which is extremely low.
Effect of Solvent on pKa Values
pKa values depend strongly on the solvent environment. Water stabilizes ions through hydrogen bonding, affecting measured acidity and basicity.
In polar aprotic solvents like DMSO and DMF, acidity constants can be significantly different. Basic species are often more reactive and easier to study there because protons behave differently than in water.
For oxide ion, some researchers suggest using crown ethers to stabilize the ion in such solvents, possibly allowing determination of pKa under non-aqueous conditions.
Summary Table of Relevant pKa Values
| Species | Equilibrium | Approximate pKa in Water | Comments |
|---|---|---|---|
| Water (H2O) | H2O ⇌ HO− + H+ | 14.0 | Widely accepted value; reflects autoionization of water. |
| Hydroxide (HO−) | HO− ⇌ O2− + H+ | >16 (unmeasurable) | Too high for direct measurement in water; reflects extremely weak acidity. |
Key Points Explaining the pKa Confusion
- The pKa 16 cited by your friend likely comes from confusion between water’s acidity and further deprotonation of hydroxide.
- Hydroxide’s pKa is less relevant as it is a base and rarely considered as an acid.
- The oxide ion is a far stronger base than hydroxide because it carries a greater negative charge, causing the conjugate acid’s pKa to be extremely high.
- Solvent has significant impact; water stabilizes ions and sets a pKa scale around 14 for water.
- Experimental pKa for oxide ion’s conjugate acid is often unmeasurable due to instability and solubility issues.
Implications for Chemistry and Practical Use
Understanding these values helps in predicting acid-base behavior and reactivity in aqueous and non-aqueous environments.
For example:
- Water’s pKa of 14 corresponds well with the neutral pH at 7, as pH = 7 is when concentrations of H+ and OH− are equal (10−7 M).
- Hydroxide’s very weak acidity means it is a strong base that rarely donates its proton but readily accepts protons, defining it as a robust base in synthesis and biochemical systems.
- Oxide ions, existing mostly outside aqueous solutions, act as extremely strong bases in solid-state chemistry and some synthetic methods.
Further Reading and Considerations
For advanced acid-base chemistry, it is important to consider:
- The impact of solvent polarity and proticity on acid-base equilibria.
- Kinetics and thermodynamics of proton transfer involving hydroxide and oxide species.
- Stabilization effects of substituents or counterions in solution.
Summary
- The pKa of water losing a proton to form hydroxide is about 14 in aqueous solution.
- Hydroxide’s further deprotonation to oxide ion has a very high, practically unmeasurable pKa (>16).
- Hydroxide is a strong base due to its negative charge, but the acidity of hydroxide (its ability to lose a proton) is very low.
- Solvent choice alters pKa and can impede or facilitate measurement of these values.
- Your friend’s value of 16 likely refers to the second deprotonation, while your intuition about hydroxide being more basic aligns with the properties of oxide ion and its very high pKa conjugate acid.
What is the pKa of hydroxide ion (HO⁻) in water?
The pKa of water, which corresponds to the H2O/HO⁻ equilibrium, is about 14 in water. This value is widely accepted and often mistaken as 15.7, but that is incorrect.
Why is the pKa for the oxide ion (O²⁻) so difficult to measure?
The oxide ion’s conjugate acid (HO⁻) has an extremely high pKa, making it hard to measure in water. Oxide ions are also poorly soluble, which complicates measurement in other solvents.
Can hydroxide be more basic than what a pKa of 16 suggests?
Hydroxide carries a -1 charge and acts as a base but not as strongly as oxide ion with a -2 charge. The pKa for oxide’s conjugate acid is much higher, so hydroxide’s basicity aligns with a pKa near 14, not above 16.
Does solvent affect the pKa values of hydroxide and oxide?
Yes. pKa values depend on the solvent used because different solvents stabilize ions differently. Oxide pKa might be measurable in solvents like DMF or DMSO, but not in water.
Why might someone guess hydroxide’s pKa to be comparable to alkanes?
Because oxide ion’s conjugate acid has an extremely high pKa, some guess it could be as high as alkanes, which are very weak acids. But this applies to oxide, not hydroxide ion.




Leave a Comment