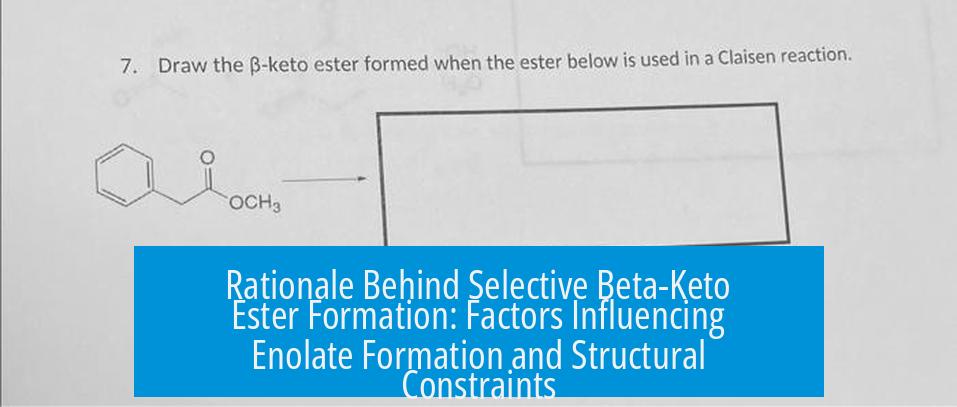
Rationale Behind Selective Beta-Keto Ester Formation

The selective formation of beta-keto esters occurs at the less substituted position primarily due to structural constraints rather than conventional kinetic or thermodynamic control of enolate formation. This selectivity defies the expectation that bases like LDA would favor enolate formation at more substituted, stable sites.
Enolate Formation and Its Limitations
Enolate chemistry usually guides beta-keto ester formation. Kinetic enolates form quickly at less substituted positions, while thermodynamic enolates favor more substituted, stable sites. However, in beta-keto ester synthesis, neither purely kinetic nor thermodynamic enolate control fully explains selectivity. If it did, strong bases such as LDA would easily direct formation toward the substituted position, yet they do not.
Structural Constraints Impacting Selectivity
Alternative explanations focus on molecular structure and steric hindrance. One key rationale involves Bredt’s rule, which states that double bonds are unfavorable at bridgehead positions in small or medium ring systems. This principle limits enolate formation at positions that would lead to bridgehead or highly strained intermediates.
Selective beta-keto ester formation at less substituted sites avoids steric clashes and unstable intermediates. Steric hindrance at more substituted positions can prevent effective enolate formation or subsequent reactions, steering the product outcome.
Summary of Influencing Factors
- Conventional kinetic and thermodynamic factors inadequately explain selectivity.
- Bredt’s rule implies structural constraints that inhibit enolate formation at certain positions.
- Steric hindrance disfavors formation at more substituted, congested sites.
- These factors combine to favor beta-keto ester formation at less substituted carbons.
Key Takeaways
- Beta-keto ester formation selectivity is largely due to structural and steric factors.
- Enolate formation mechanisms alone do not predict the observed site selectivity.
- Bredt’s rule limits unfavorable enolate intermediates at bridgehead positions.
- Understanding these constraints aids rational synthesis planning in organic chemistry.
Why does the beta-keto ester form selectively at the less substituted position?
The beta-keto ester forms at the less substituted position due to structural and steric factors rather than the usual enolate formation preferences.
Does kinetic or thermodynamic control explain this selective formation?
Kinetic and thermodynamic enolate formation do not significantly influence this selectivity. If they did, bases like LDA would easily produce the observed outcome.
What alternative explanations exist beyond enolate chemistry?
Structural constraints, such as those linked to Bredt’s rule, may limit enolate formation at more substituted, bridgehead positions. This restricts the reaction to less hindered sites.
How does Bredt’s rule affect beta-keto ester formation?
Bredt’s rule prevents forming stable double bonds at bridgehead carbons. This rule may block enolate formation at certain positions, steering the reaction toward less substituted sites.
Are steric factors important in selective beta-keto ester formation?
Yes, steric hindrance can restrict enolate formation at bulky or highly substituted positions, favoring the creation of beta-keto esters at less hindered sites.



Leave a Comment