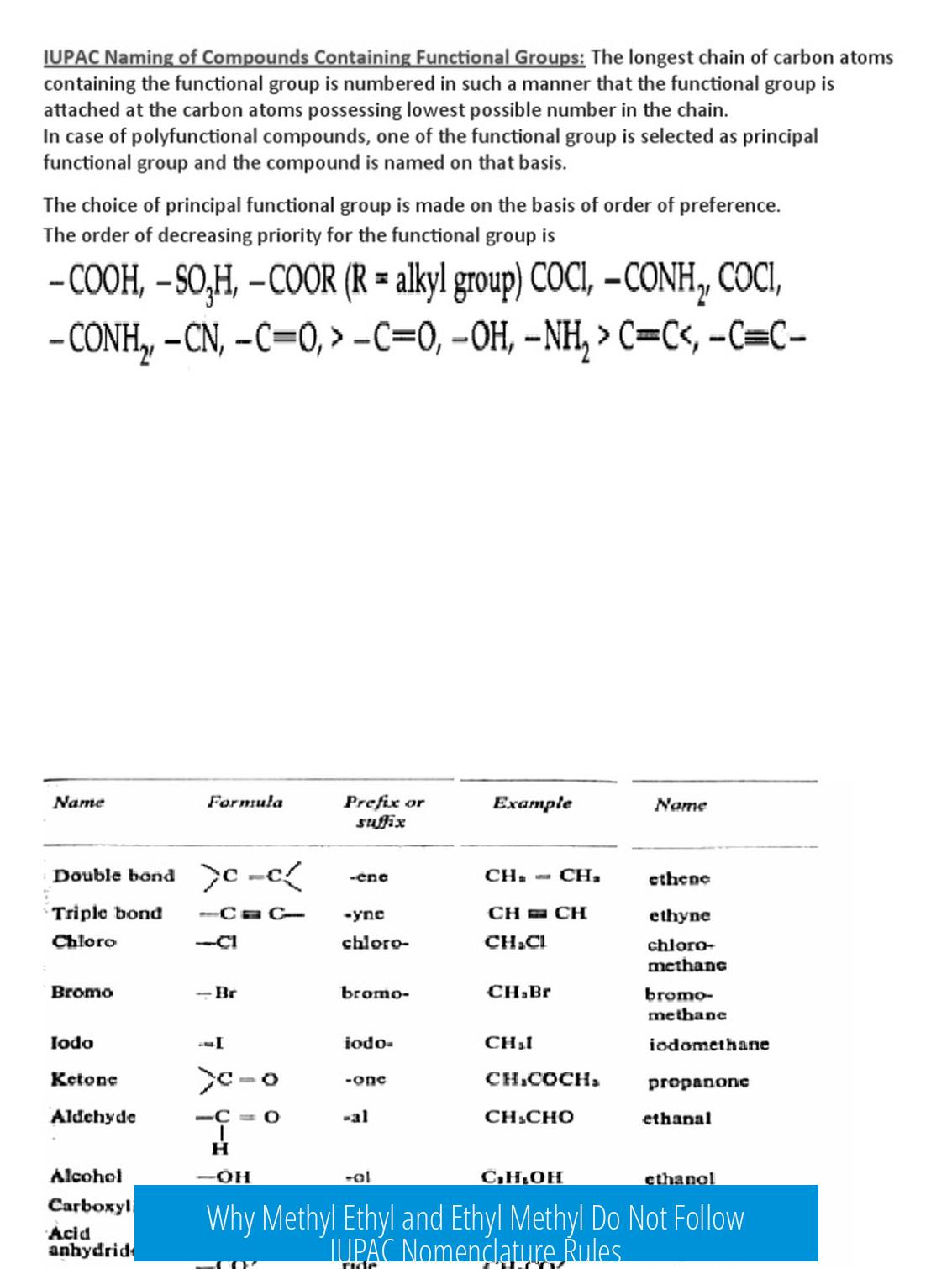
Why Does (Methyl Ethyl) vs (Ethyl Methyl) Not Follow IUPAC Nomenclature?

The key reason is that the naming depends on identifying the substituent group by its longest carbon chain rather than simply listing substituents in arbitrary order. “Methyl ethyl” or “ethyl methyl” is not a straightforward IUPAC name of a free molecule but refers to substituent nomenclature, which uses specific rules.
Substituent Naming Rules
- IUPAC nomenclature places the substituent before the parent chain name.
- When naming substituents, the longest continuous chain in the substituent group acts as its “parent.”
- For example, an ethyl group with a methyl attached at the 1-position is named based on the ethyl chain with a 1-methyl substituent.
Longest Chain Determines Substituent Name
In the name “1-methylethyl,” the ethyl group is regarded as the parent chain. The substituent methyl group attaches to this at carbon 1, so the entire substituent is methylethyl.
This naming follows IUPAC guidelines because substituent groups must be named by their longest carbon chain starting from the carbon attached to the main molecule.
Difference from Naming a Free Molecule
If the compound were a free molecule rather than a substituent group, it would be named methylethane, reflecting the methane unit attached to ethane.
Thus, simply saying “methyl ethyl” or “ethyl methyl” does not represent a proper IUPAC name of a molecule but rather a substituent naming logic where the longest chain is prioritized.
| Term | Description | Example |
|---|---|---|
| Substituent Name | Longest carbon chain in substituent acts as parent | 1-methylethyl |
| Free Molecule Name | Named by overall longest chain and substituents on main chain | Methylethane |
Summary of Key Points
- IUPAC nomenclature prioritizes the longest carbon chain in substituent groups.
- “Methyl ethyl” or “ethyl methyl” as names do not directly follow IUPAC for free molecules.
- “Methylethyl” refers to a substituent named by an ethyl chain with a methyl at the 1-position.
- Proper IUPAC names must distinguish whether the group is a substituent or a parent molecule.
Why is “methylethyl” the correct IUPAC name for the substituent rather than “methyl ethyl”?
In IUPAC naming, the substituent is named after its longest carbon chain, which is ethyl here. A methyl group is attached to the 1-position on the ethyl chain, forming methylethyl, following IUPAC rules.
Does “methyl ethyl” violate IUPAC naming rules?
“Methyl ethyl” is not the standard IUPAC name for the substituent. It reverses the order and does not reflect the longest chain in the substituent. IUPAC requires naming based on the longest carbon chain in the substituent group first.
How does the substituent naming differ from naming a free molecule like methylethane?
The substituent is named starting from the R-C carbon of the group attached to the main molecule. For a free molecule, methylethane is correct. For a substituent, the name focuses on the longest chain, making methylethyl the proper name.
Why does the order of “methyl” and “ethyl” matter in these names?
IUPAC naming prioritizes the longest carbon chain in a substituent. “Ethyl” precedes “methyl” when the ethyl chain is the base structure. This order clarifies the main backbone and attached groups.
Can “ethyl methyl” ever be a correct IUPAC name?
No, “ethyl methyl” does not follow IUPAC conventions when naming substituents because it implies the methyl is the parent chain, which is shorter. The longest chain must come first, making “methylethyl” correct.



Leave a Comment