Mass is an important concept in physics and is defined as the amount of matter present in an object, body or system. It has a standard unit of measurement, the kilogram (kg). Mass can be found by measuring an object’s weight and applying calculations to the results. This can be done through a simple formula, which is moles = mass ÷ molar mass, or n = m ÷ M.
The importance of mass and its measurement cannot be overstated, as it helps us to understand the physical properties of the world around us. It also helps us to calculate the amount of energy, momentum, and other properties of an object or system. Knowing the mass of an object is also important in understanding its gravitational pull, as well as in determining its density.
In this article, we will discuss the concept of mass and how to measure it. We will also learn about the different ways of finding mass, and the different steps involved in doing so. We will also explore the different types of mass, such as the mass number and molar mass. Finally, we will look at some interesting facts about mass and its importance in the physical world. So, let’s get started!
How do you find mass 1?
Mass is a fundamental concept in physics and is a measure of the amount of matter in an object or body. Mass is often confused with weight, but they are not the same. Mass is a measure of the amount of matter an object contains, while weight is a measure of the force of gravity on that object. Knowing how to calculate mass and weight can be a useful tool for understanding the world around us.
What is Mass?
Mass is the amount of matter present in any object or body. Everything we see around us has mass, from tables and chairs to footballs, glasses, and even air. Mass is what makes an object light or heavy. It is measured in kilograms (kg).
How to Calculate Mass
If the mass of an object is given in different units, such as milligrams (mg), micrograms (μg), or kilograms (kg), it can be converted to grams (g). To do this, divide the mass by 1,000 if it is given in milligrams or by 1,000,000 if it is given in micrograms, or multiply it by 1,000 if it is given in kilograms.
How to Calculate Weight
We can also calculate the weight of something if we know its mass. Weight is usually referred to in pounds in everyday life, but in physics, the standard unit of this force is the Newton (N). To calculate the weight of something, multiply its mass (in kilograms) by the gravitational force (in Newtons).
Weight = Mass x Gravitational Force
Calculating Moles from Mass
We can also calculate the number of moles (n) from the mass (m) of a substance by using the following equation:
Moles = Mass ÷ Molar Mass
n = m ÷ M
Examples
Let’s look at some examples of how to calculate mass and weight.
If we have an object with a mass of 10 mg, we can convert it to grams by dividing it by 1,000, giving us a mass of 0.01 g. If we know the gravitational force is 9.8 N, we can calculate the weight of the object by multiplying the mass by the force, giving us 0.098 N.
If we have a substance with a mass of 500 μg, we can convert it to grams by dividing it by 1,000,000, giving us a mass of 0.0005 g. If we know the molar mass of the substance is 30 g/mol, we can calculate the number of moles present in the substance by dividing the mass by the molar mass, giving us 0.00016 moles.
In conclusion, mass is a measure of the amount of matter in an object or body and is measured in kilograms (kg). Weight is a measure of the force of gravity on an object and is measured in Newtons (N). Knowing how to calculate mass and weight can be a useful tool for understanding the world around us. We can also calculate the number of moles present in a substance by dividing the mass by the molar mass.
We hope this article has been helpful in understanding the concept of mass and how to calculate it. If you have any questions or comments, please leave them in the comments section below.
How do you find the mass of a number?
The mass number of an atom or isotope is the sum of the number of protons and neutrons in its nucleus. Knowing the mass number of an atom is essential for understanding its chemical makeup and behavior. So, how do you find the mass number?
Atomic Mass and Mass Number
The atomic mass of an element is the average of all of its isotopes’ masses. The mass number of an isotope is the sum of the number of protons and neutrons in its nucleus. Subtracting an element’s mass number from its atomic mass tells you the number of protons in its nucleus.
Finding the Mass Number
The best place to look for an element’s atomic mass number is in the periodic table. It’s displayed under the symbol for the element. For example, the atomic mass number of carbon is 12, which means that it has 12 protons and 12 neutrons. However, some elements can have more than one isotope with different mass numbers, so it’s important to double-check the element’s atomic mass number before calculating its mass number.
Using the Mass Number
Once you’ve found an element’s mass number, you can use it to calculate the number of neutrons in the nucleus. To do this, subtract the atomic number from the element’s mass number. The atomic number of an element is the number of protons it has in its nucleus. For example, if an element has a mass number of 16, and its atomic number is 8, then the number of neutrons in its nucleus is 8 (16-8 = 8).
Calculating Mass
The mass of an atom can be calculated using the mass number and the element’s atomic mass. To calculate the mass, simply multiply the mass number by the atomic mass of the element. For example, if an element has a mass number of 16 and an atomic mass of 12, then the mass of the element is 192 (16 x 12 = 192).
Knowing the mass number of an atom is essential for understanding its chemical makeup and behavior. The mass number of an atom or isotope can be found by looking at the periodic table and subtracting the atomic number from the element’s mass number. Once you have the mass number, you can use it to calculate the number of neutrons in the nucleus and the mass of the atom.
How do you find the mass number of?
The mass number of an atom or isotope is the sum of the number of protons and the number of neutrons in its nucleus. This number can be used to identify the element and to calculate its atomic mass. Knowing the mass number of an atom can be helpful in understanding the properties of the element and its chemical behavior.
What is a Mass Number?
The mass number of an atom is a measure of the total number of protons and neutrons in its nucleus. It is equal to the sum of the protons and neutrons, and is sometimes referred to as the atomic mass number. The mass number of an element is indicated by its symbol in the periodic table, and is usually written as a superscript after the element symbol. For example, the mass number for carbon is 12, which is indicated by the symbol C12.
How to Calculate the Mass Number
The mass number of an atom can be calculated by adding the number of protons and the number of neutrons in its nucleus. The number of protons is equal to the element’s atomic number, which is indicated by its position in the periodic table. The number of neutrons can be determined by subtracting the element’s atomic number from its mass number.
The Importance of Knowing the Mass Number
Knowing the mass number of an atom can be helpful in understanding the properties of the element and its chemical behavior. Different elements can have different mass numbers, and the mass number can affect the properties of the element. For example, carbon can have different isotopes, which are atoms of the same element with different mass numbers. The mass number of an isotope affects the stability of the element and its ability to form chemical bonds.
The best place to look for an element’s mass number is in the periodic table. It is usually indicated by its symbol, and is written as a superscript after the element symbol. For example, the mass number for carbon is 12, which is indicated by the symbol C12.
The mass number of an atom can also be determined by subtracting the element’s atomic mass from its mass number. This will tell you the number of protons in its nucleus. Additionally, the mass number can be calculated by adding the number of protons to the number of neutrons.
The mass number of an atom or isotope is the sum of the number of protons and the number of neutrons in its nucleus. Knowing the mass number of an atom can be helpful in understanding the properties of the element and its chemical behavior. The mass number of an element is usually indicated by its symbol in the periodic table, and can be calculated by adding the number of protons and the number of neutrons in its nucleus.
How do you find the exact molar mass?
When it comes to chemistry, understanding the molar mass of an element is essential. The molar mass, also known as molecular weight, is the mass of one mole of a substance. It’s a measure of the size of the molecule and is used to calculate the number of particles in a given mass.
What is the molar mass?
The molar mass is the mass of one mole of a substance. It’s usually expressed in grams per mole, or g/mol. The molar mass is determined by the atomic mass of the element, which can be found on the periodic table.
For example, if the atomic mass of sulfur (S) is 32.066 amu, then its molar mass is 32.066 g/mol. Through the use of dimensional analysis, scientists can use the molar mass to convert between mass, number of moles and number of atoms.
How is the molar mass calculated?
The molar mass of an element is calculated by multiplying the atomic mass of the element by Avogadro’s number (6.02 x 1023 particles/mole). This number is a constant that tells us how many particles are present in one mole of any substance.
For example, if the atomic mass of carbon (C) is 12.0107 amu, then the molar mass of carbon is 12.0107 g/mol. To calculate the molar mass of a compound, you must take the atomic mass of each element in the compound and multiply it by Avogadro’s number.
How do you use the molar mass?
The molar mass is useful for a variety of calculations. For example, it can be used to calculate the number of moles in a given mass. It can also be used to calculate the mass of a given number of moles.
The molar mass can also be used to determine the amount of energy released or absorbed in a chemical reaction. The amount of energy released or absorbed is determined by the difference in the molar masses of the reactants and products.
The molar mass of any element can be determined by finding the atomic mass of the element on the periodic table. By recognizing the relationship between the molar mass (g/mol), moles (mol), and particles, scientists can use dimensional analysis to convert between mass, number of moles and number of atoms very easily.
Having a thorough understanding of the molar mass of an element and how to calculate it is essential for any chemist. Knowing how to calculate and use the molar mass can help chemists understand the nature of a given substance and aid in predicting the outcome of a chemical reaction.
What are the steps to measure mass?
Mass is an important concept in chemistry, biology, and other sciences, as it is used to quantify the amount of matter in an object. Mass measurements are typically performed using a balance, which is a device that uses the acceleration of Earth’s gravity to measure mass. To accurately measure mass, two methods can be used on most instruments: subtraction and taring.
Subtraction Method
The subtraction method involves subtracting the mass of the empty container from the mass of the container with the material inside. This approach is used when the container is too large or heavy to be tared. To use this method, the weight of the empty container should be measured first and then the container should be filled with the material you are measuring. Finally, the weight of the full container should be measured and the difference between the two measurements should be calculated.
Taring Method
The taring method is the most common way to measure mass. This technique involves resetting the balance to zero before measuring the mass of the material. To use this method, the balance should be reset to zero and then the material should be placed in the container and the mass should be measured. This method is preferred when the container is small enough to be tared.
Other Instruments
In addition to laboratory balances, there are other instruments that can be used to measure mass. For example, a spring scale can be used to measure the weight of an object. A spring scale works by measuring the amount of force that is applied to a spring. The force is then converted into a measurement of mass.
Another type of instrument used to measure mass is an electronic scale. These scales use a variety of sensors, such as strain gauges, to measure the mass of an object. The data from the sensors is then processed by a computer to calculate the mass.
Mass measurements are an important concept in the sciences and are typically performed using a balance. There are two main methods used to measure mass: subtraction and taring. In addition to laboratory balances, there are other instruments, such as spring scales and electronic scales, that can be used to measure mass. Regardless of the instrument used, accurate measurements of mass are essential for a variety of scientific experiments.
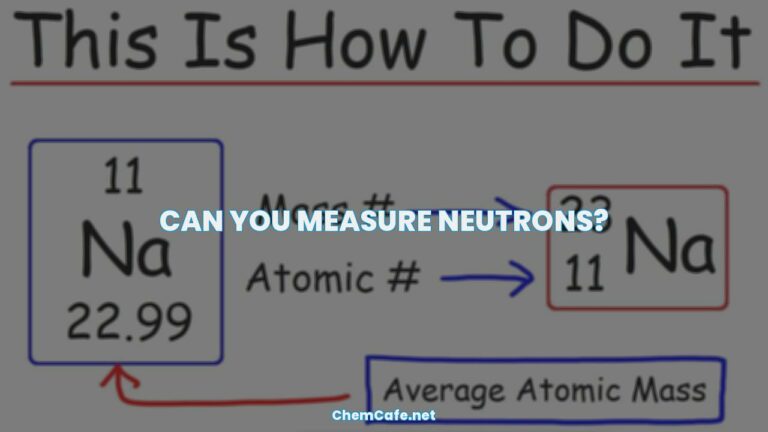
What is your mass number?
When we talk about atoms, we often refer to an element’s mass number. But what exactly is the mass number? In this blog post, we’ll explain what the mass number is and how it can be calculated.
What is Mass Number?
The mass number is a superscript written on the left side of the element symbol. It is the total number of protons and neutrons in an atom. The mass number of an atom or isotope can be calculated by adding together the number of protons (atomic number) and the number of neutrons.
Mass number = atomic number + number of neutrons
For instance, if we look at the first six elements of the periodic table in Table 1, we can see that the mass number for helium is 4, which is equal to its atomic number (2) plus the number of neutrons (2).
Variants of Mass Number
The mass number for all elements is usually a whole number, though there are some exceptions. For instance, He-4 has a mass number 4 and an atomic number of 2. However, it exists in more variants, such as He-2, He-3, He-5, He-6, He-7, He-8, He-9, and He-10. These variants have the same atomic number but a different mass number.
How to Calculate Mass Number
Calculating the mass number of an atom is fairly simple. All you need to do is add the number of protons and the number of neutrons together. This is because the mass number is the sum of the protons number and the neutrons number in its nucleus.
For example, if we look at the element carbon, we can see that it has an atomic number of 6 and a mass number of 12. This means that it has 6 protons and 6 neutrons in its nucleus.
In conclusion, the mass number is the total number of protons and neutrons in an atom. It can be calculated by adding the number of protons and the number of neutrons together. It is usually a whole number, though there are some exceptions. Knowing the mass number of an element can help you better understand its characteristics and properties.


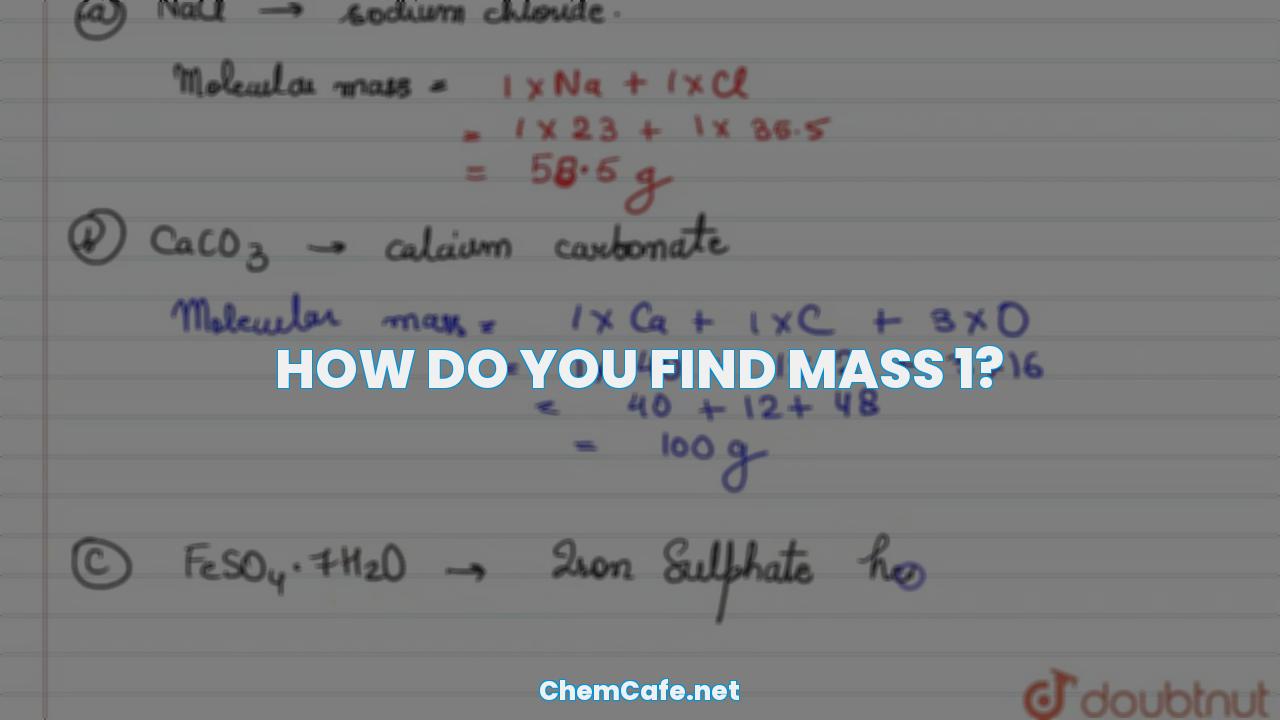

Leave a Comment