CRISPR/Cas and Cancer Treatment
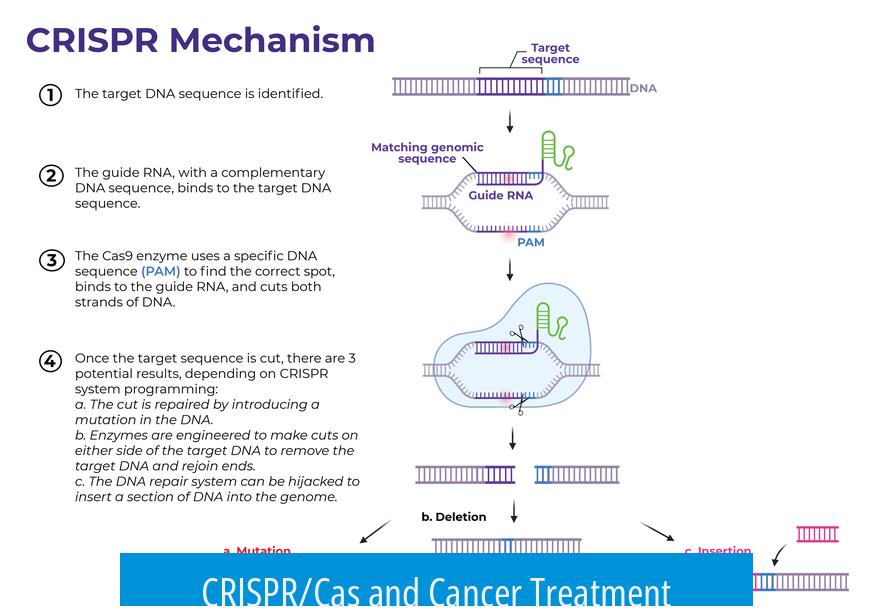
CRISPR/Cas offers promising avenues for cancer treatment, but challenges in delivery, accuracy, and safety limit its current clinical use.
Delivery Efficiency of Cas9 to Cancer Cells
The delivery of Cas9 enzyme into cancer cells is a major hurdle. This process is not fully efficient and typically does not reach every cancer cell. For example, eliminating 95% of cancer cells still allows surviving cells to grow back. Improving delivery methods is critical to achieve better treatment outcomes and prevent tumor relapse.
Off-Target Activity and Accuracy of Cas9
Cas9’s precision in editing DNA is not perfect. Even a small percentage of off-target activity can cause unintended mutations in millions of healthy cells. This risk of off-target mutagenesis poses safety concerns for therapies since mutated healthy cells might lead to new malignancies or other disorders.
CRISPR in Oncogene Manipulation for Research
CRISPR/Cas systems are valuable tools in cancer research. Scientists use them to knock out oncogenes or induce mutations to study cancer development. These applications help in understanding the molecular mechanisms behind tumor growth and testing potential drug targets. Cas proteins can directly remove mutated genes without relying on apoptotic enzymes.
Current Research and Human Trials
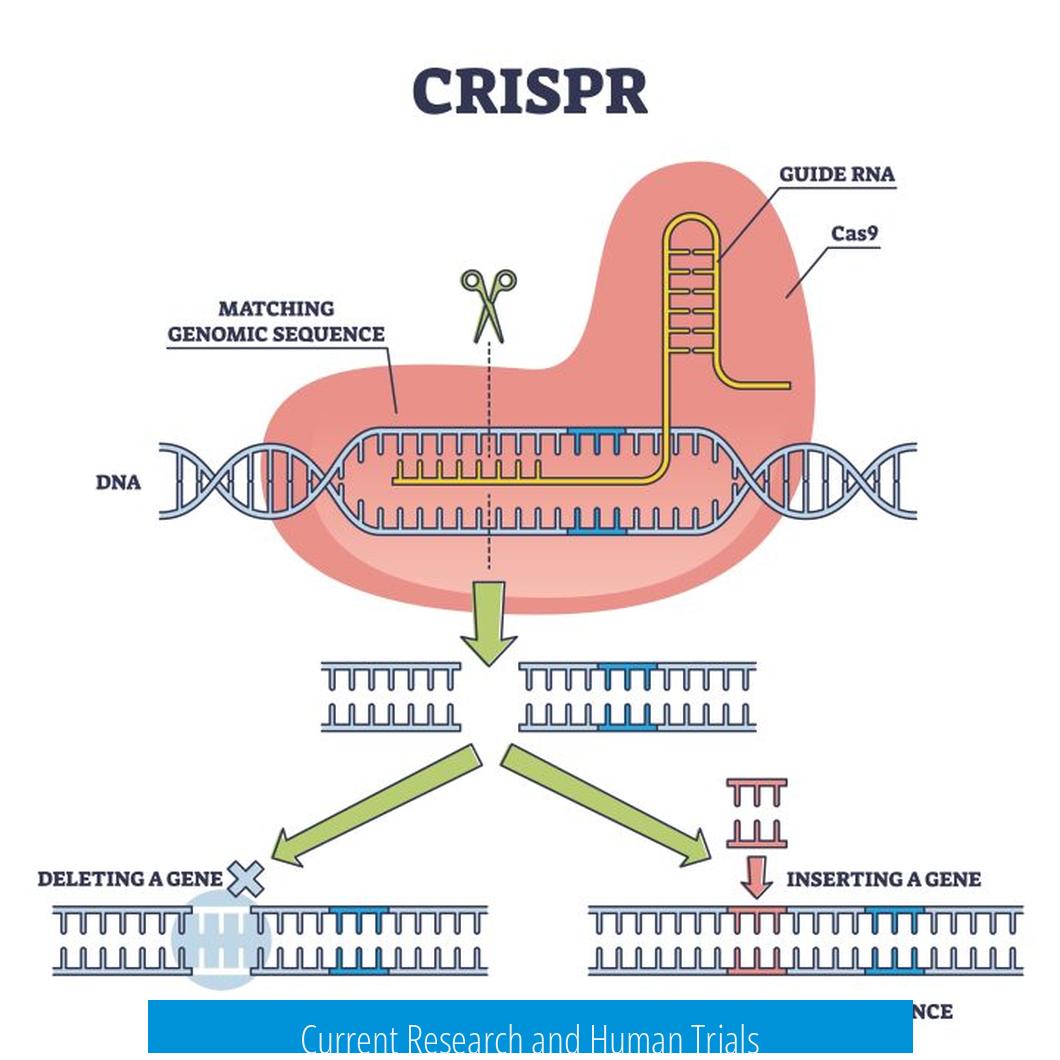
Research efforts aim to translate CRISPR/Cas9 into clinical cancer therapies. Human trials exploring CRISPR’s therapeutic potential are ongoing. However, many uncertainties remain, such as immune system reactions, insertional mutagenesis risks, and variable editing efficiency. Each of these factors introduces technical challenges that need resolution before widespread clinical use.
Summary of Key Points
- Cas9 delivery into cancer cells lacks complete efficiency, limiting therapeutic effectiveness.
- Off-target gene editing by Cas9 raises safety concerns due to possible unintended mutations.
- CRISPR serves as a powerful tool in oncogene research and gene function studies.
- Human clinical trials face challenges including immune response, mutation risks, and delivery control.
- Further optimization is required for safe, effective CRISPR-based cancer treatments.
CRISPR/Cas and Cancer Treatment: The Cutting Edge’s Sharpest Tool or a Double-Edged Sword?
So, what is CRISPR/Cas doing in cancer treatment? Simply put, it acts like molecular scissors that can cut or alter cancer genes to stop tumor growth or help researchers understand cancer better. Yet, the journey from lab bench to bedside is filled with hurdles. Let’s explore the fascinating—and sometimes tricky—world of CRISPR/Cas in the fight against cancer.
Breaking Down the Delivery Dilemma: Getting Cas9 Inside Cancer Cells
Imagine delivering a life-saving package, but only 95% of the parcels reach the right address. That’s one big challenge with CRISPR. The Cas9 enzyme, the star editor of the CRISPR system, must reach cancer cells to cut faulty DNA.
Here’s the kicker: delivery is not 100% efficient. As one expert points out, “If you only kill 95% of the cancer cells, the rest will just grow and replace the dead ones.” This means even an almost perfect hit can leave room for cancer to sneak back. Cancer is sneaky, and incomplete editing means the fight isn’t over yet.
Scientists are experimenting with all sorts of delivery methods—viral vectors, nanoparticles, even microscopic bubbles that pop open inside cells—to improve this. But efficiency is still a work in progress.
The Off-Target Quandary: When Precision Cuts Miss Their Mark
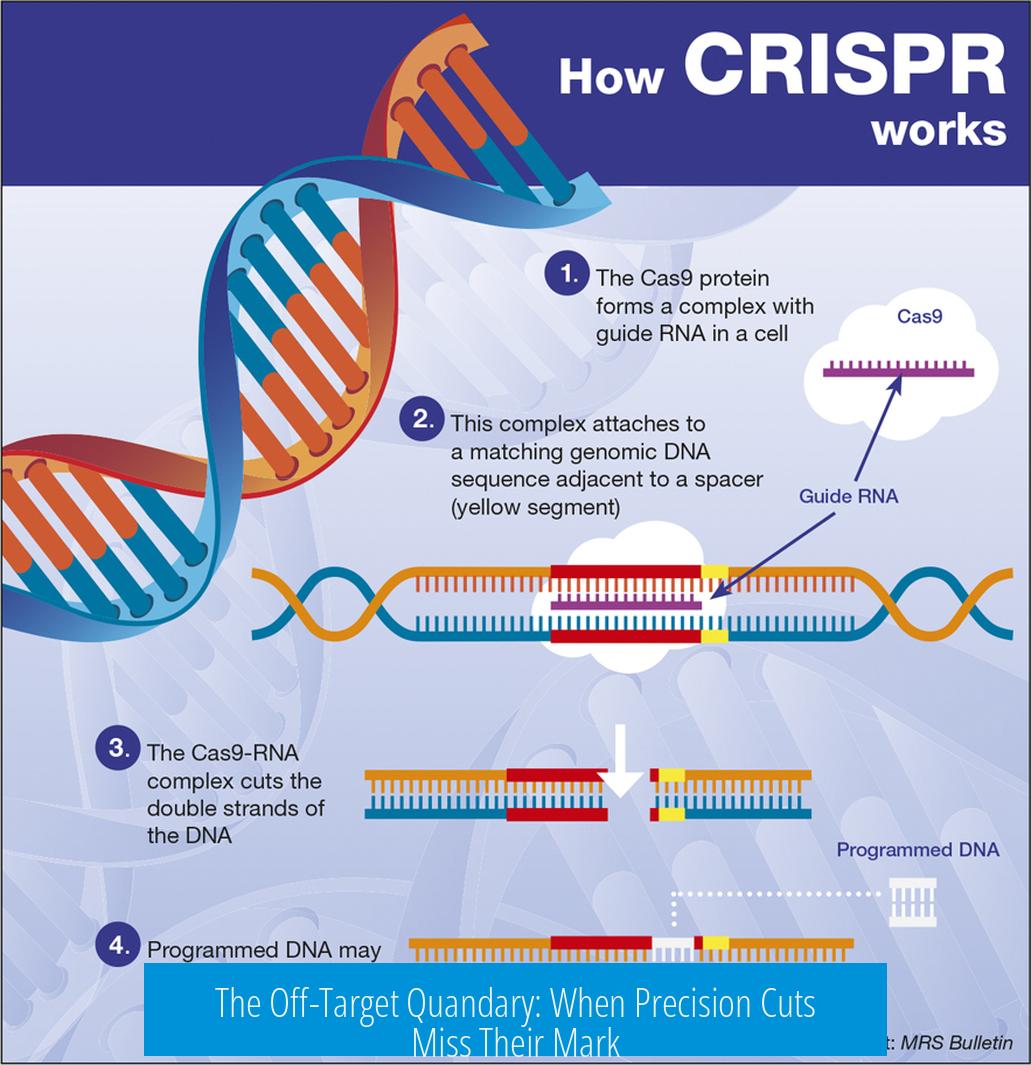
CRISPR’s appeal lies in its precision, but perfection remains elusive. Cas9 sometimes cuts DNA where it shouldn’t. Off-target mutagenesis means healthy cells can be accidentally edited, possibly triggering mutations or other unwanted effects.
Picture this: delivering Cas9 to a billion cells with 0.1% off-target activity results in a million potentially mutated “innocent bystander” cells. This isn’t just a statistic; it’s a serious safety concern.
This off-target activity raises the question—how can we trust the editor to edit rightly? Scientists are tinkering with engineered Cas9 variants and improved guide RNAs that aim to boost accuracy. Yet, these tools must be proven safe in humans before wide use.
Not Just Treatment: CRISPR Powers Cancer Research by Altering Oncogenes
Beyond treatment, CRISPR is a powerhouse in cancer research. Researchers can knock out oncogenes—genes that cause cancer when mutated—or switch them on to study how tumors grow.
Unlike traditional gene editing tools that require extra enzymes like caspases, CRISPR’s Cas proteins can directly remove mutated genes. This streamlines experiments and speeds discovery.
Think of it as a rapid-fire toolkit that lets scientists explore cancer’s playbook in detail. This knowledge feeds back into better treatments and personalized medicine strategies.
Human Trials and the Road Ahead: A Trailblazing Journey with Unknowns
CRISPR’s arrival in clinical trials for cancer is thrilling but comes with big question marks. Researchers grapple with uncertainties about treatment efficiency, immune system reactions, unintended insertions of genetic material, and the overall risk of new mutations.
One insider admits, “Every technical problem is a flaw, because we cannot control them”. This cautious approach slows progress but ensures patient safety stays front and center.
Despite these challenges, ongoing trials across the world carefully test CRISPR’s potential. The field is dynamic, and improvements happen quickly. Future breakthroughs may turn current limitations into stepping stones.
Why Should You Care About CRISPR and Cancer Now?
Here’s the deal—cancer remains a leading cause of death. New tools like CRISPR bring hope of more precise, personalized ways to target tumors unlike broad chemotherapy or radiation.
But this isn’t a magic bullet yet. The technology demands respect, understanding, and tons of research.
For patients and families, keeping an eye on CRISPR developments can provide hope without unrealistic expectations. For scientists and healthcare professionals, it means balancing enthusiasm with responsibility.
Summing It Up: CRISPR’s Promise Is Vast, But Caution Rules For Now
- Delivery efficiency is impressive but incomplete—missing a small fraction can let cancer rebound.
- Off-target effects raise safety fears due to possible unintended mutations in healthy cells.
- CRISPR boosts research by precisely turning oncogenes on or off, speeding understanding of cancer mechanisms.
- Human trials proceed carefully to address unknowns about immune responses and mutation risks before mainstream use.
So, while CRISPR/Cas is transforming how we think about cancer, it’s not yet a simple fix. The science is exhilarating. The risks urge caution. The promise is enormous.
As this gene-editing saga unfolds, wouldn’t it be great to witness a future where cutting out cancer is as routine as cutting hair? For now, CRISPR stands as one of medicine’s sharpest tools—if only we perfect the delivery and accuracy to match its edge.
What limits the success of CRISPR/Cas9 in killing all cancer cells?
Delivery of Cas9 to cancer cells is not fully efficient. Even if 95% of cancer cells are killed, the remaining cells can grow and replace the dead ones, reducing treatment effectiveness.
How does off-target activity of Cas9 affect cancer treatment safety?
Cas9 is not perfectly accurate. Small percentages of off-target effects could mutate healthy cells, potentially causing new problems as millions of healthy cells might be affected during treatment.
How is CRISPR used in cancer research related to oncogenes?
CRISPR helps knock out or activate oncogenes in cancer cells. This allows researchers to study gene function without needing extra proteins like caspases to remove mutated genes.
What challenges exist in applying CRISPR to human cancer treatments?
- Low delivery efficiency
- Immune system reactions
- Risk of harmful gene insertions
- Uncertain mutation probabilities
These issues create technical flaws that complicate human trials.




Leave a Comment