The atom is the smallest unit of matter, and it is composed of a nucleus and electrons that orbit around it. Electrons are the negatively charged particles that make up most of an atom’s mass and determine its chemical behavior. Electrons have different levels of energy, which are referred to as shells. These shells are numbered from one to seven, and the number of electrons in each shell determines the chemical properties of the atom.
The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He). Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell.
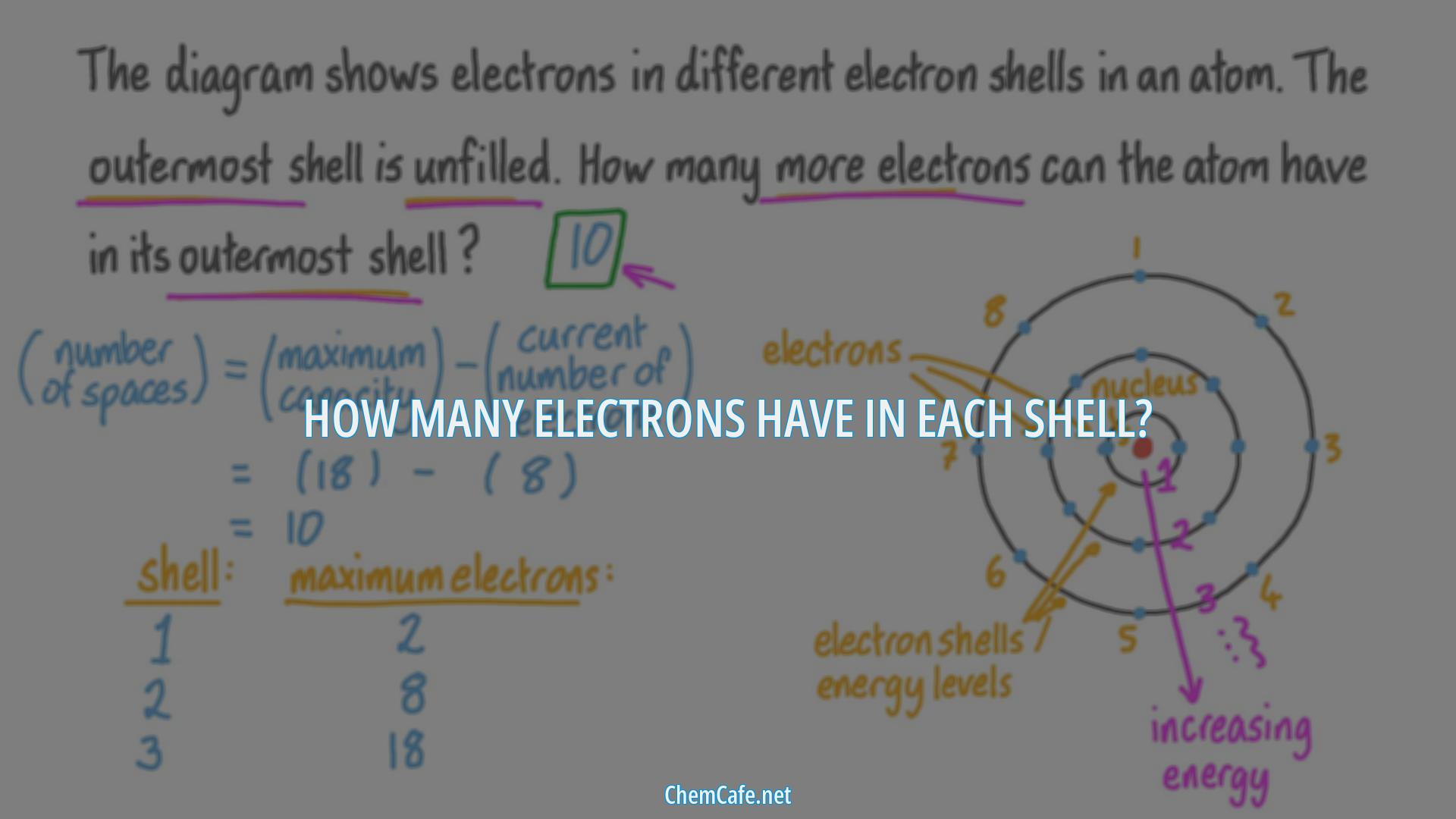
Each successive electron shell can only hold a certain number of electrons. Higher energy shells can hold more electrons. The first shell can only contain two electrons, the second eight and the third eighteen. Electrons in the same shell have the same amount of energy. This means that the second shell can hold a maximum of eight electrons (2+6=8). Notice that there are eight elements in the second row of the periodic table. It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react (be gained, lost, or shared). You might imagine that, if two atoms bumped into each other, it would be the outer electrons that would interact first.
Understanding and keeping track of the number of electrons in each shell can help us to better understand the behavior of atoms, and can also help us to predict the properties of different elements. For example, elements in the same group of the periodic table will have the same number of electrons in their outer shell. This is why elements of the same group have similar chemical properties. By understanding how electrons interact with each other and how they are distributed in different shells, we can better understand the behavior of atoms, and ultimately, the behavior of matter.
How many electrons have in each shell?
Atoms are composed of protons, neutrons, and electrons. The electrons are arranged in shells around the nucleus of the atom. Each of these shells can hold a certain number of electrons, and the number of electrons in each shell determines the chemical properties of an atom. So, how many electrons have in each shell?
The First Shell
The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He). Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell.
Maximum Number of Electrons in Each Shell
Each successive electron shell can only hold a certain number of electrons. Higher energy shells can hold more electrons. The first shell can only contain two electrons, the second eight and the third eighteen. Electrons in the same shell have the same amount of energy.
This means that the second shell can hold a maximum of eight electrons (2+6=8). Notice that there are eight elements in the second row of the periodic table. It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react (be gained, lost, or shared). You might imagine that, if two atoms bumped into each other, it would be the outer electrons that would interact first.
Electron Shells and Chemical Reactions
The number of electrons in the outer shell of an atom will determine how it behaves in a chemical reaction. If the outer shell is full, then the atom is not likely to react. However, if the outer shell is not full, then the atom is more likely to react. This is because atoms with incomplete outer shells tend to gain, lose, or share electrons to achieve a full outer shell.
Summary
The number of electrons in each shell determines the chemical properties of an atom. The first shell can only hold two electrons, the second eight, and the third eighteen. Electrons in the same shell have the same amount of energy. It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react. The number of electrons in the outer shell of an atom will determine how it behaves in a chemical reaction.
How many electrons does each shell?
Atoms are made up of electrons, which orbit the nucleus of the atom at different ranges. These ranges are called shells, and each shell has a different energy level.
The number of electrons that can fit in each shell is determined by the principal quantum number, which can range from 1 to 7. The closer the shell is to the nucleus, the lower the energy level and the fewer electrons it can hold.
The First Shell
The first shell, which is closest to the nucleus and has the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. That’s why there are only two elements in the first row of the periodic table (hydrogen and helium).
The Second Shell
The second shell has two subshells: 2s and 2p. The 2s subshell can hold up to two electrons and the 2p subshell can hold a maximum of six. That means the second shell can hold a total of eight electrons.
The Third Shell
The third shell has three subshells: 3s, 3p, and 3d. The 3s subshell can hold up to two electrons, the 3p subshell can hold a maximum of six, and the 3d subshell can hold a maximum of ten. That means the third shell can hold a total of eighteen electrons.
Higher Energy Shells
As you move to higher energy shells, the number of electrons they can hold increases. For example, the fourth shell can hold a maximum of thirty-two electrons, the fifth shell can hold fifty, and the sixth shell can hold seventy-two.
Electrons in the Same Shell Have the Same Amount of Energy
It’s important to note that electrons in the same shell have the same amount of energy. That’s why each shell can hold a maximum of a certain number of electrons.
In summary, the first shell can only contain two electrons, the second eight and the third eighteen. Higher energy shells can hold more electrons. Electrons in the same shell have the same amount of energy.
Understanding the number of electrons in each shell can help you better understand the behavior of atoms and how they interact with each other. This knowledge can be used to form molecules and other compounds, which can be used to create a wide range of products.
How many electron shells are in each shell?
Atoms are composed of electrons, protons, and neutrons, and the arrangement of these particles determines the chemical and physical properties of the atom. Electrons orbit the nucleus at different distances, which are known as shells. Each shell has a different energy level, and the number of electrons it can hold is determined by its energy level. The energy level of each shell is given a number called the principal quantum number, or n.
What is an electron shell?
An electron shell is the outermost part of an atom around the nucleus. It is composed of one or more electron subshells, or sublevels. Electron shells are given their name from the Bohr model, where electrons are believed to orbit the nucleus at certain distances, forming “shells”. Each shell is associated with a particular range of electron energy, and it must be filled completely before electrons can be added to an outer shell. The electrons in the outermost shell are responsible for the atom’s chemical properties (known as the valence shell).
How many electron shells are in each shell?
The number of electron shells in each shell is determined by the principal quantum number, n. The closest shell has a value of n=1, and the next shell has a value of n=2, and so on. The number of electrons in each shell is determined by the amount of energy it contains, and the maximum number of electrons in each shell follows the formula 2n².
For example, the first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold a maximum of 8 electrons, and the third shell (n=3) can hold a maximum of 18 electrons. The fourth shell (n=4) can hold a maximum of 32 electrons, and the fifth shell (n=5) can hold a maximum of 50 electrons.
What happens when a shell is filled?
When a shell is filled, the electrons in the outermost shell determine the chemical properties of the atom. This is because the outer shell is the only part of the atom that interacts with other atoms. When the outermost shell is filled, the atom is said to be in its “ground state” and it is stable and non-reactive. If an atom has an incomplete outer shell, it can gain, lose, or share electrons with other atoms, which can make it chemically reactive.
In conclusion, electron shells are the outermost part of an atom around the nucleus. The number of electron shells in each shell is determined by the principal quantum number, n, and the maximum number of electrons in each shell follows the formula 2n². When a shell is filled, the electrons in the outermost shell determine the chemical properties of the atom.
How many electrons are in a K shell?
Atoms contain electrons in different energy levels or shells, and the number of electrons in each shell can vary. The K shell is the innermost shell, and it is the first energy level of an atom. It can contain a maximum of two electrons.
Understanding the structure of an atom requires knowledge of the electron shells, and how many electrons can occupy each one. The K shell is the innermost shell and it is the first energy level of an atom. It can contain a maximum of two electrons.
What is an electron shell?
An electron shell is the part of an atom around its nucleus. It is a group of atomic orbitals with the same value of the principal quantum number (n). Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed “shells”.
How many electrons can be in a K shell?
The maximum number of electrons that can be present in a K shell is two. This is because of the Pauli exclusion principle, which states that no two electrons can have the same set of quantum numbers. Since the K shell has only one quantum number, the maximum number of electrons that can occupy it is two.
Why is it important to know how many electrons are in a K shell?
It is important to know how many electrons are in a K shell because the number of electrons in the K shell determines the properties of an atom. For example, the number of electrons in the K shell determines the reactivity of an atom. Atoms with two electrons in their K shell are more reactive than atoms with only one electron in their K shell.
What is the next energy level after the K shell?
The next energy level after the K shell is the L shell. The L shell can contain up to eight electrons. After the L shell, the next energy level is the M shell, which can hold up to eighteen electrons.
Knowing the number of electrons in each shell is important for understanding the structure and properties of atoms. The K shell is the innermost shell and it can contain a maximum of two electrons. The L shell can contain up to eight electrons, and the M shell can hold up to eighteen electrons. Understanding the number of electrons in each shell can help us understand the properties of atoms and how they interact with each other.
How do you find electrons per shell?
Electrons are the negatively charged particles that form the outer shell of an atom. Knowing the number of electrons per shell can be important for understanding how atoms interact with each other and how they form chemical bonds. To calculate the number of electrons per shell, you will need to know the principal quantum number (n) associated with each shell.
What is the Principal Quantum Number (n)?
The principal quantum number, or n, is a number that describes the energy level of an electron shell in an atom. It is related to the distance of the electron from the nucleus of the atom and increases as you move away from the nucleus. The principal quantum number is a whole number, starting at 1 for the closest shell to the nucleus, and increasing as you move away.
How Many Electrons Fit in Each Shell?
The number of electrons that can fit in each shell depends on the principal quantum number. A general rule is that the maximum number of electrons per shell is equal to two times the principal quantum number squared. For example, the maximum number of electrons in the first shell (n=1) is 2 x (1 x 1) = 2. The maximum number of electrons in the second shell (n=2) is 2 x (2 x 2) = 8.
How to Calculate Electrons Per Shell
Once you know the principal quantum number for the shell you are interested in, you can calculate the total number of electrons per shell. To do this, subtract the number of electrons in the shells with lower principal quantum numbers from the maximum number of electrons for the shell you are interested in.
For example, if you want to calculate the number of electrons in the third shell (n=3), you would subtract the number of electrons in the first two shells (2 electrons for n=1 and 8 electrons for n=2) from the maximum number of electrons for n=3, which is 2 x (3 x 3) = 18. So, the total number of electrons in the third shell is 18 – 2 – 8 = 8.
The number of electrons per shell can be calculated by knowing the principal quantum number associated with that shell and subtracting the number of electrons in the shells with lower principal quantum numbers. This calculation is important for understanding how atoms interact with each other and how they form chemical bonds. Knowing the number of electrons per shell can be a useful tool for chemists and physicists alike.
How many electrons can 2nd shell hold?
The second shell of electrons in an atom is the second layer of energy surrounding the nucleus. It is the second-lowest energy state and can hold a maximum of 8 electrons. This is one of the most important concepts in chemistry because it helps explain why elements in the same column of the periodic table have similar properties.
Understanding how many electrons can be held by the second shell is essential in understanding the chemical properties of an element. It is also important for understanding the structure of molecules. Understanding the number of electrons in each shell helps us predict the reactivity of atoms and molecules and the stability of chemical bonds.
How Many Electrons Are in Each Shell?
The number of electrons in each shell is determined by the amount of energy the electrons have. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He).
Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell. Each successive electron shell can only hold a certain number of electrons. Higher energy shells can hold more electrons. The first shell can only contain two electrons, the second eight and the third eighteen. Electrons in the same shell have the same amount of energy.
This means that the second shell can hold a maximum of eight electrons (2+6=8). Notice that there are eight elements in the second row of the periodic table. It is only the electrons in the outer-most shell, called the VALENCE shell, that tend to react (be gained, lost, or shared). You might imagine that, if two atoms bumped into each other, it would be the outer electrons that would interact first.
How Does the Number of Electrons in the Second Shell Affect Reactivity?
The number of electrons in the valence shell determines the reactivity of an element. The valence shell can be full, partially full, or empty. Elements with full valence shells are usually the least reactive. A full valence shell is usually made up of eight electrons, which is the same as the number of electrons in the second shell.
Elements with partially-filled valence shells tend to be more reactive because they can gain or lose electrons in order to fill their valence shells. Elements with empty valence shells tend to be the most reactive because they can easily gain electrons to fill their valence shells.
The second shell of electrons in an atom holds a maximum of 8 electrons. This is an important concept in chemistry because it helps explain why elements in the same column of the periodic table have similar properties and why the valence shell determines the reactivity of an element. Understanding the number of electrons in each shell is essential in understanding the structure of molecules and the stability of chemical bonds.



Leave a Comment