RNA Strand Folding via Intramolecular Hydrogen Bonds
It is normal and possible for an RNA strand to be long enough to form hydrogen bonds between its own bases, resulting in bending and complex secondary structures that critically affect eukaryotic cell functions.
Formation of RNA Secondary Structure

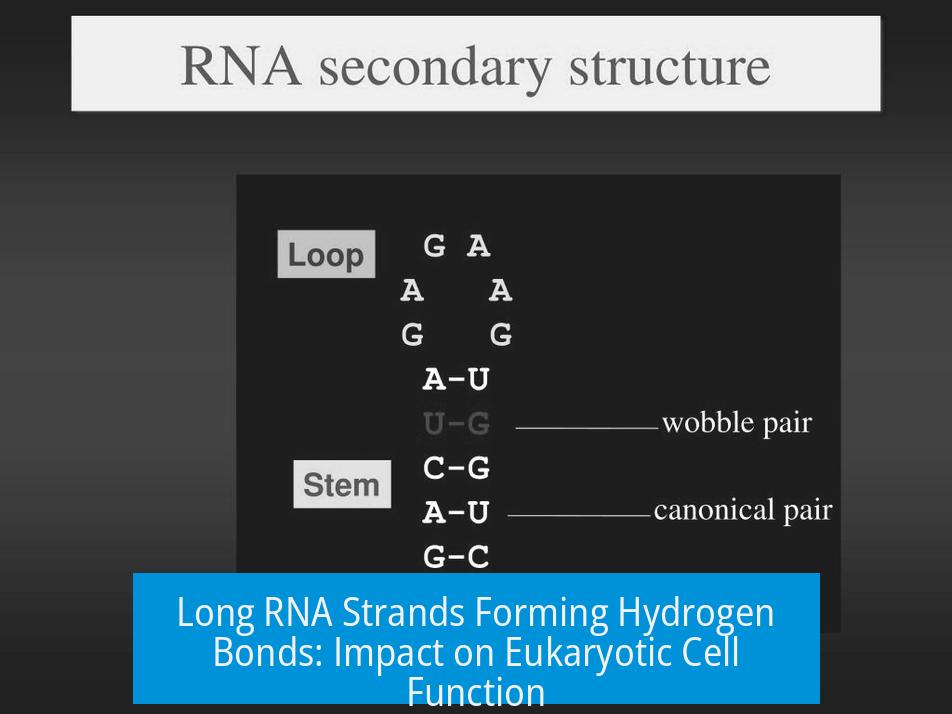
Though RNA is often referred to as single-stranded, this description is misleading. In reality, RNA molecules longer than a few bases almost always fold back on themselves. This folding occurs through hydrogen bonds formed between complementary bases within the same strand, creating loops, hairpins, and other secondary structures.
These structures cause bending in the RNA backbone, producing intricate shapes essential for RNA’s functional roles within the cell. Such folding occurs naturally and is a fundamental characteristic of most cellular RNAs.
Biological Role of RNA Secondary Structures in Eukaryotic Cells
Secondary structures formed by intramolecular hydrogen bonding have several important functions in eukaryotic cells:
- Mediation of RNA-protein interactions critical for processes like splicing and translation.
- Enabling catalytic activities in ribozymes, RNA molecules with enzymatic function.
- Formation of transfer RNA (tRNA) and ribosomal RNA (rRNA) structures essential for protein synthesis.
- Supporting internal ribosome entry sites on messenger RNA (mRNA), which modulate translation initiation.
Examples and Evolutionary Insight
RNA aptamers exemplify how long RNA molecules fold into specific three-dimensional shapes by forming internal hydrogen bonds. This folding generates functional sites for binding proteins or small molecules.
The folding and catalytic abilities of RNA secondary structures underpin the RNA World Hypothesis. This theory suggests that early RNA-based life relied on these intrinsic structures for genetic information storage and enzymatic activity before protein enzymes evolved.
Summary of Key Points
- Long RNA strands normally fold by intramolecular hydrogen bonding, causing bending and stable secondary structures.
- These structures are essential for RNA’s diverse roles in eukaryotic cellular processes.
- RNA folding allows proper interaction with proteins, catalysis, and translation mechanisms.
- The RNA World Hypothesis highlights the ancient evolutionary significance of such structural features.
- RNA aptamers are practical examples demonstrating constant RNA folding through self-base pairing.
Is It Normal for Long RNA Strands to Bend by Forming Hydrogen Bonds Within Themselves? And Does This Affect Eukaryotic Cell Function?
Yes, it is not only normal, but expected that RNA strands longer than a few bases form hydrogen bonds among their own bases, causing bending and complex folding. This self-pairing, or secondary structure, plays a critical role in how eukaryotic cells function. But before we jump to cell biology’s grand drama, let’s break it down step-by-step and make sense of this molecular origami.
Ever heard that RNA is single-stranded? It’s a classic oversimplification. When we say “single-stranded,” we mean RNA doesn’t have a complimentary strand paired like DNA does. However, this doesn’t imply the molecule is a limp noodle. Far from it! RNA tends to fold back on itself extensively, creating hydrogen bonds among its bases. This causes bending, loops, hairpins, and quite complex 3D structures collectively known as RNA secondary structure.
The Science Behind the RNA Folding Party
Hydrogen bonds are like little social magnets for nucleotides. The adenine, uracil, guanine, and cytosine bases within the RNA strand seek each other out, pairing up internally wherever they can. This self-pairing snuggles the strand into folds and bends rather than leaving it as a taut linear chain.
Basically, the longer the RNA, the more opportunity it has to play matchmaker with its own bases. The folding isn’t accidental or disorderly—it’s a purposeful, highly regulated event critical for the RNA to perform its functions properly.
Why Does RNA Folding Matter in the Cell? Spoiler: It’s Vital
At this point, you might wonder, “So what if it folds? Does it really impact how cells work?” The answer is a loud YES.
The secondary structure enables RNA to interact with proteins and even act as enzymes themselves. Consider transfer RNA (tRNA) and ribosomal RNA (rRNA): without their distinct shapes created by intramolecular hydrogen bonds, protein synthesis would halt right at step one.
For example, tRNAs are responsible for ferrying amino acids to the ribosome, and their cloverleaf folding pattern comes directly from this self-bonding. Similarly, rRNAs fold into intricate shapes that form the ribosome’s catalytic core, making it the protein assembly line in every eukaryotic cell.
And there’s more – messenger RNA (mRNA) molecules hide little treasure troves of structured elements, like internal ribosome entry sites (IRES) that allow translation to start even under stressful conditions. These IRES depend on the RNA’s ability to fold back on itself correctly.
RNA Enzymes: The Ribozymes Show How Folding Powers Catalysis
Think enzymes are just proteins’ turf? Not really. Ribozymes are RNA molecules that fold into precise shapes, thanks to these internal hydrogen bonds, allowing them to catalyze chemical reactions. This functionality relies entirely on their secondary structure. Without the bends and loops formed by hydrogen bonding, RNA’s enzymatic magic wouldn’t happen.
The Bigger Picture: Evolution Loves RNA Folding
Let’s take a tiny detour back in time. The RNA World Hypothesis proposes that early life may have relied solely on RNA not just as genetic material but also as catalysts, because of these folding capabilities. RNA molecules millions of years ago formed secondary structures enabling enzymatic activities crucial for primitive life.
This evolutionary perspective highlights how internal base pairing and strand bending are not just molecular quirks—they’re foundational to biology as we know it.
Practical Example: RNA Aptamers—Nature’s Custom Folded Sensors
Modern biotechnology loves these folding principles. RNA aptamers are artificially synthesized strands designed to bind specific targets, like a lock-and-key mechanism. They achieve shape specificity through intramolecular hydrogen bonding—exactly the bending we’ve been discussing.
This is a shining example of how the natural folding property of RNA is harnessed for practical applications, like diagnostics or targeted drug delivery.
Does This Folding Ever Go Wrong? And What About Eukaryotic Cell Function?
Since RNA secondary structure is essential, cells maintain strict quality control over RNA folding and processing. Misfolded RNA can disrupt protein synthesis or fail in regulatory roles, impacting cell health. Thankfully, cells have RNA-binding proteins and molecular chaperones that assist proper folding.
In eukaryotic cells, where the complexity is high, these folding behaviors are intimately tied to gene expression, signaling, and metabolism. So, RNA strand bending due to hydrogen bonds isn’t just normal—it’s a biological necessity. It affects everything from how genes are read to how proteins are made, directly influencing cell function and organismal health.
Summary Table: RNA Strand Length & Folding – What Happens?
| RNA Strand Length | Structural Behavior | Biological Role |
|---|---|---|
| Few bases (short) | Minimal folding | Limited function, often regulatory |
| Moderate length | Secondary structure formation via H-bonds | Mediates RNA-protein interactions, regulatory roles |
| Long strands | Complex folds, hairpins, loops, extensive bending | Functions in tRNA, rRNA, ribozymes, mRNA translation control |
What Should You Take Away?
If you ever hear that RNA is just a simple single strand, gently correct that person. RNA’s beauty lies in its ability to self-fold, creating functional shapes through hydrogen bonds that cause bending. These shapes make RNA indispensable in eukaryotic cell biology—from building proteins to regulating genes.
So yes, it’s normal and necessary that long RNA strands fold and bend thanks to internal hydrogen bonding. This folding shapes life at the molecular level, steering countless essential processes inside every eukaryotic cell.
Now, next time someone says RNA is just a single strand, you can impress them with the elegant truth: RNA is a dynamic origami artist, folding, bending, and turning to keep life running smoothly.
Is it common for long RNA strands to fold back on themselves and form hydrogen bonds internally?
Yes. Long RNA strands naturally form intramolecular hydrogen bonds between their bases. This causes the strand to bend and create secondary structures.
How does the secondary structure of RNA impact eukaryotic cell functions?
Secondary structures mediate RNA-protein interactions and enable enzymatic activities of ribozymes. They are essential for tRNA, rRNA, and mRNA functions in the cell.
Can these RNA folds affect the efficiency of protein synthesis in eukaryotic cells?
Yes. Proper folding allows internal ribosome entry sites to function correctly, influencing translation initiation and overall protein synthesis efficiency.
Do these RNA intramolecular hydrogen bonds have any evolutionary significance?
They do. The RNA World Hypothesis suggests early RNA genomes used such folding to act as enzymes, supporting genetic functions before DNA and proteins evolved.
Are there practical examples where RNA folding through internal hydrogen bonding is crucial?
RNA aptamers are a key example. They form specific 3D shapes via internal hydrogen bonds to bind target molecules selectively, demonstrating functional RNA folding in action.



Leave a Comment