QIAGEN Ni-NTA Agarose Protocol for His-Tagged Protein Purification
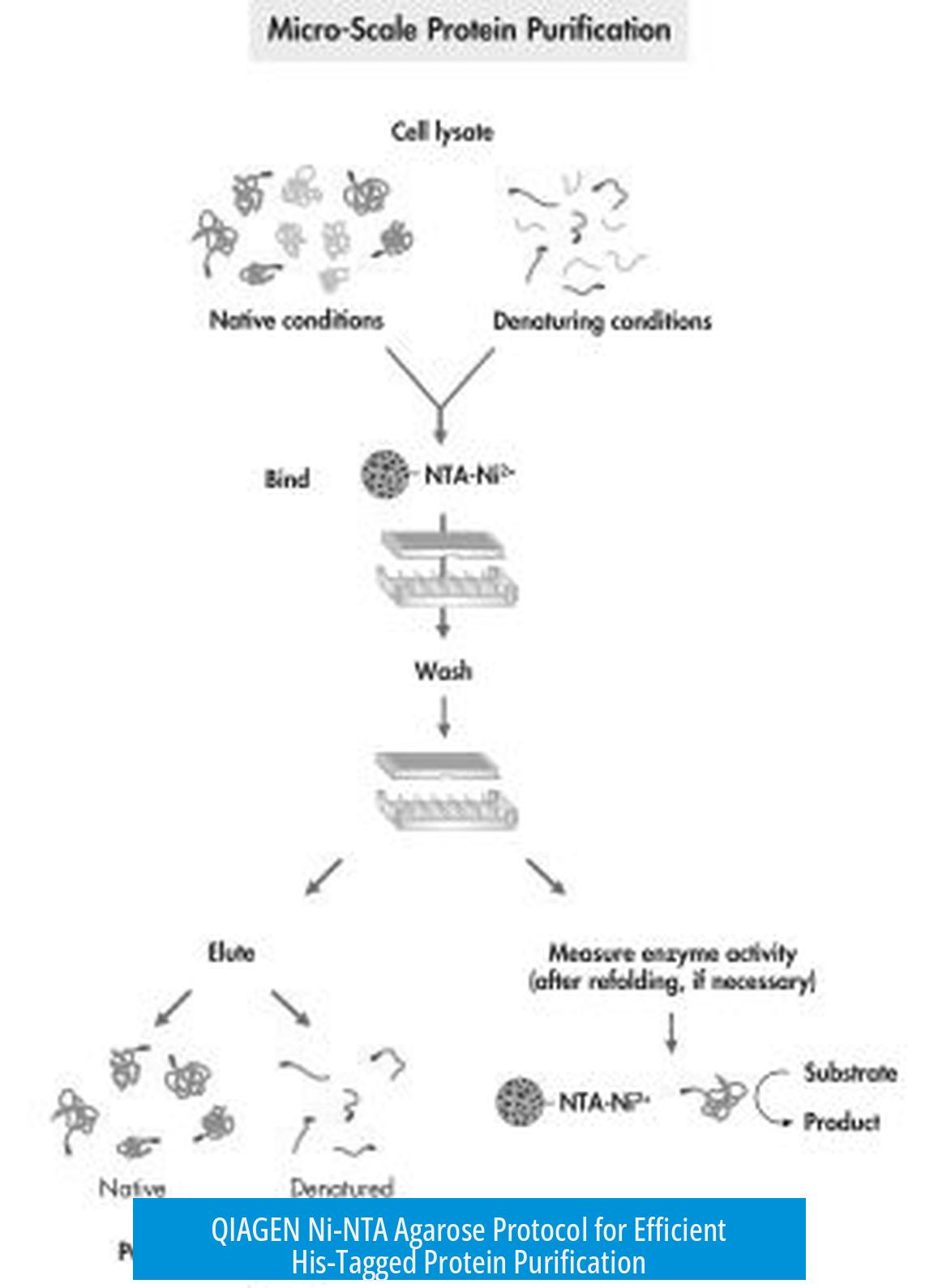
The QIAGEN Ni-NTA agarose protocol isolates His-tagged proteins using nickel-charged agarose beads. The method relies on the specific binding of histidine residues to Ni-NTA groups on the beads. There are key workflow choices: either pre-mix beads with lysate or pass lysate through a packed bead column. Both approaches yield effective purification, with procedural variations depending on experimental needs.
Binding His-Tagged Proteins to Ni-NTA Agarose

The core step involves the interaction of His-tags on target proteins with Ni-NTA agarose beads. Two main methods exist:
- Incubate beads and lysate together before loading into a column.
- Load beads first and then pass the lysate over the packed beads in a column.
Pre-mixing increases the contact time between beads and His-tags. This practice generally improves binding efficiency and is often preferred.
Optimizing Contact Time and Binding Efficiency
Longer contact allows more efficient binding of His-tagged proteins to Ni-NTA agarose. Pre-mixing beads with lysate enhances this step.
After incubation, the bead-lysate mixture can be poured into a column for subsequent washing and elution steps.
Washing and Elution Steps
Following binding, washes remove nonspecific proteins and impurities. Washing is performed within the column.
The protocol supports passing the cleared lysate over packed beads directly. Optionally, the flow-through can be collected and re-applied to the column to maximize protein capture.
Protocol Flexibility and Customization
This protocol adapts to different experimental goals. For example, researchers purifying nanobodies from periplasmic extracts may tweak the procedure based on their sample type.
Both pre-mixing and column passing yield good results. Choice depends on sample amount, time constraints, and protein characteristics.
Key Takeaways
- His-tagged proteins bind specifically to Ni-NTA agarose beads.
- Pre-mixing beads and lysate enhances binding by increasing contact time.
- Lysate can be passed over pre-packed columns as an alternative workflow.
- Washing steps occur in-column to remove impurities.
- Protocol allows customization for specific protein purification needs.
Mastering the QIAGEN Ni-NTA Agarose Protocol: A Fresh Look at His-Tag Protein Purification
So, how does the QIAGEN Ni-NTA agarose protocol actually pull off the purification of His-tagged proteins? The magic happens when His tags bind to the Ni-NTA agarose beads. You have two roads to travel: mix them first or pour your sample through a packed column. Either way, the goal is tight binding, cleaner protein, and happier experiments.
If that sounds simple, it is! But the nuances make your results shine or flounder. Ready to dig into the details?
Grabbing Your Protein by the His-Tag Collar
His-tagged proteins are like VIP guests wearing shiny badges—they can get special treatment with Ni-NTA agarose beads. These beads have nickel ions tightly held by nitrogen atoms in the NTA (nitrilotriacetic acid) structure. The histidine residues on the tag love to bind the nickel. It’s as if the His-tag throws a party and only those beads have the best snacks.
Two ways to get this party started:
- Mix beads and lysate together: Incubate the beads with your sample before loading onto the column.
- Load lysate onto a packed bead column: Pour your sample onto beads already sitting in a column.
Interestingly, pre-mixing leads to a longer interaction time, which translates to stronger binding. Imagine shaking hands for longer—it sticks better! This is why many pros prefer the pre-mixing method. It’s incremental but impactful.
The Charm of Contact Time: Why It Matters
Think of binding efficiency like a speed date. The longer you spend chatting, the better the connection. When beads and lysate incubate together, you maximize the chance that each His-tagged protein finds its nickel partner. Simply passing your sample quickly through a column might not give enough time for all proteins to latch on.
This longer contact time can be a game-changer when purified protein yield matters. So, if you’re after maximum yield and purity from your His-tagged sample, pre-mixing is your friend.
Washing Away the Unwanted Bystanders
Once your proteins have hooked up with the beads, the next vital step is washing. You want to wash away the flakes—the proteins and impurities that didn’t RSVP properly to your party. By mixing beads and lysate first, binding increases, which makes your washing tasks smoother and more effective.
When you run wash buffers through your column, contaminants are flushed out while the His-tagged proteins remain firmly attached. This step ensures your final product isn’t just pure but pristinely pure.
Protocol Variations: Finding Your Flow
Here’s a fun bit: QIAGEN’s protocol isn’t cast in stone. Labs customize it depending on their workflow, sample types, and even quirks.
Pre-mixing lysate with beads before column loading holds the reputation of being more efficient for many.
However, the alternative method—loading lysate onto an already packed column—can still hold value.
If you run the flow-through a second time through the column, you can boost yield by catching proteins that didn’t bind initially.
It’s like casting a wider net on your fishing trip. More protein caught, less lost to the ocean of unbound molecules.
Customization: The Key to Nailing Your Niche
Protocol flexibility is more than just optional—it’s a must. One researcher working on periplasmic purification of nanobodies tails their protocol to fit that niche. How? They might tweak incubation times, buffer compositions, or washing steps.
Why care about this? Because no two proteins or experimental setups are identical. Your protocol should bend, not break.
The best approach is to start with the QIAGEN recommended protocol. Try both pre-mixing and direct loading. Then, fine-tune based on your yields and purity assessments.
Practical Tips to Elevate Your QIAGEN Ni-NTA Experience
- Prioritize contact time: Pre-mixing beads and lysate enhances binding. A 30-minute incubation works well for most samples.
- Consider flow-through reprocessing: If you notice low yield, collect your flow-through and run it through the column again to recover leftovers.
- Customize washing stringency: More washes or slightly higher imidazole concentrations can improve purity without losing target proteins.
- Check your pH: Maintain pH around 7.4-8.0 for optimal His-tag and Ni-NTA interaction.
Wrapping Up: Is the QIAGEN Ni-NTA Agarose Protocol Worth the Hype?
Absolutely. It offers reliable, high-purity His-tag protein purification with room for adaptation. Whether you’re a newbie or an experienced scientist, this protocol’s flexibility suits various experimental designs.
In short, pre-mixing beads with lysate improves binding through increased contact time, leading to a cleaner product and better yield. Then, you wash away unwanted guests, and depending on workflow, you can either use the pre-mixed slurry or pass lysate over beads packed in a column—potentially running the flow-through twice for an extra protein boost.
Feeling inspired to tweak and perfect your own QIAGEN Ni-NTA agarose setup? Every sample is a new puzzle. Your customized protocol will be the key to solving it.
Q1: How does pre-mixing His-tagged proteins with Ni-NTA agarose beads affect binding?
Pre-mixing the beads and lysate increases contact time. This improves binding efficiency before applying the mixture to the column.
Q2: Can I use the flow-through from Ni-NTA purification again to increase protein yield?
Yes, you can collect the flow-through when lysate passes through a packed column and run it through the column a second time to improve yield.
Q3: Is it necessary to follow the same Ni-NTA agarose protocol for all protein types?
No, the protocol is customizable. For example, periplasmic nanobody purification may require slight adjustments to fit specific workflows.
Q4: What are the two main approaches for applying lysate to Ni-NTA agarose beads?
- Incubate lysate and beads together then load onto the column.
- Pour cleared lysate directly over packed beads in the column.
Q5: Why might someone prefer incubating beads and lysate before column loading?
This increases the contact time between His-tagged proteins and beads, enhancing binding before any washing steps.




Leave a Comment