Have you ever wondered what exactly the electron configuration of an atom is? Electron configurations are an important part of understanding the nature of atoms and how they interact with each other. It is the arrangement of electrons into shells that has the most effect on chemical properties, so it is important to understand the basics of electron configuration.
To start, we need to know what an electron is. An electron is a subatomic particle with a negative electrical charge and is the fundamental particle of electricity. In an atom, electrons are arranged into shells, which are energy levels around the nucleus of the atom. Each shell can hold a different number of electrons, and the maximum number of electrons that a shell can hold is determined by its subshell.
The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Each added electron is assigned to the lowest-energy sublevel available.
Let’s take a look at some examples of electron configurations. The electron configuration of Si (atomic number 14) is 1s2 2s2 2p6 3s2 3p2. The electron configuration of Mg (atomic number 12) is 1s2 2s2 2p6 3s2. The electron configuration for the element aluminum, which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1.
The electron configuration of 1s2 2s2 2p6 3s1 is incorrect, as Mg (atomic number 12) has the correct electron configuration of 1s2 2s2 2p6 3s2. The electron configuration of 1s2 2s2 2p6 3s2 3p4 is the electron configuration of the element silicon (Si), while the electron configuration 1s2 2s2 2p6 3s2 3p6 is the electron configuration of the element sulfur (S). The electron configuration of 1s2 2s2 2p2 is the electron configuration of the element helium (He), while the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is the electron configuration of the element iron (Fe). Finally, the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is the electron configuration of the element cobalt (Co).
In conclusion, understanding the basics of electron configuration is important in order to understand the nature of atoms and how they interact with each other. Knowing the electron configuration of a particular atom can help us understand its chemical and physical properties.
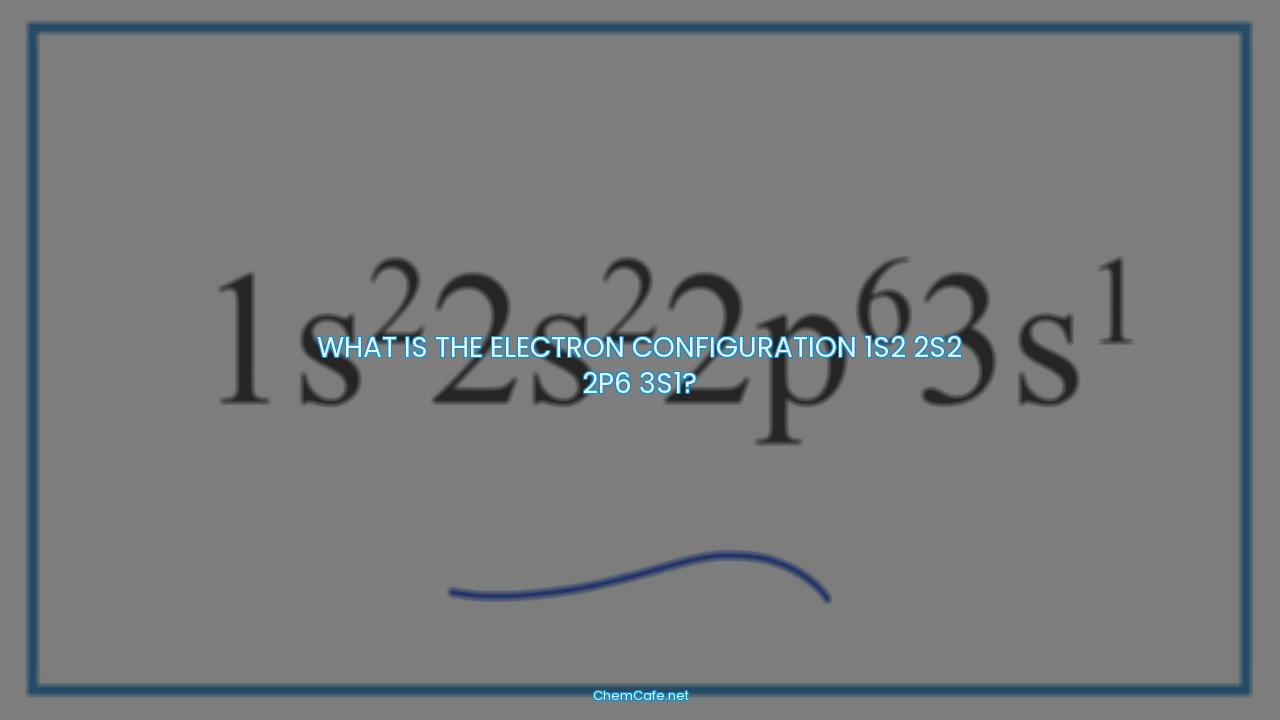
What is the electron configuration 1s2 2s2 2p6 3s1?
The electron configuration of an atom is a representation of the number of electrons in each sublevel of each energy level of the atom. In order to determine the electron configuration of an atom, one must start at the nucleus and add electrons one-by-one until the number of electrons equals the number of protons in the nucleus.
Subshells and Maximum Electron Capacity
Each added electron is assigned to the lowest-energy sublevel available. Different subshells have a different maximum number of electrons that can occupy them. For example, the s subshell can hold up to 2 electrons; the p subshell can hold up to 6 electrons; the d subshell can hold up to 10; and the f subshell can hold up to 14.
Shells and Their Effect on Chemical Properties
It is the arrangement of electrons into shells that has the most effect on chemical properties. Thus, the focus of most discussions on electron configuration is on the shells. To make it easier to refer to the shells, numbers are used to indicate the shell in which an electron is located.
The Electron Configuration of 1s2 2s2 2p6 3s1
Now let’s consider the electron configuration 1s2 2s2 2p6 3s1. This is incorrect as it does not correspond to the number of protons in either silicon (Si, atomic number 14) or magnesium (Mg, atomic number 12). The correct electron configuration for Si is 1s2 2s2 2p6 3s2 3p2, and the correct electron configuration for Mg is 1s2 2s2 2p6 3s2.
In conclusion, the correct electron configuration of Mg is 1s2 2s2 2p6 3s2, and the incorrect electron configuration 1s2 2s2 2p6 3s1 does not correspond to either Si or Mg. Understanding the electron configuration of atoms is important for understanding the chemical behavior of elements.
What is the electron configuration 1s2 2s2 2p6 3s2 3p4?
Electron configurations are used to describe the arrangement of electrons in an atom. They can help to explain the behavior of atoms in chemical reactions and are also useful for predicting the properties of elements. The electron configuration for an atom is typically written as a series of numbers representing the orbitals in which electrons are located.
Introduction to Electron Configurations
An electron configuration is a numerical representation of the distribution of electrons around the nucleus of an atom. Each orbital is written with a number representing the principal quantum number (n) followed by a letter representing the angular momentum quantum number (l). The letter is associated with the type of orbital, s, p, d, and f. The number before the letter indicates the shell the electron is in.
For example, a 1s orbital is a s orbital in the first shell. A 2p orbital is a p orbital in the second shell. The maximum number of electrons that can be in each orbital is two. The electron configuration for an atom is written as a series of numbers representing the orbitals in which electrons are located.
What is the Electron Configuration 1s2 2s2 2p6 3s2 3p4?
The electron configuration 1s2 2s2 2p6 3s2 3p4 is the configuration for the element phosphorus. This element has 15 electrons, which are arranged in the following way: two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and four electrons in the 3p orbital.
Phosphorus is a non-metal element in group 15 of the periodic table. It has five valence electrons and is commonly found in the form of phosphate minerals. It is a very reactive element and is used in many industrial applications. Phosphorus is essential for life, as it is a component of DNA and RNA.
The electron configuration 1s2 2s2 2p6 3s2 3p4 is the configuration for the element phosphorus. Phosphorus is a non-metal element in group 15 of the periodic table and has five valence electrons. It is a very reactive element and is essential for life, as it is a component of DNA and RNA. Understanding the electron configuration of an element can help to explain the behavior of atoms in chemical reactions and can also be used to predict the properties of elements.
What has the electron configuration 1s2 2s2 2p6 3s2 3p6?
The electron configuration 1s2 2s2 2p6 3s2 3p6 is a shorthand notation of the arrangement of electrons around the nucleus of an atom. This arrangement is also known as the electronic structure of the atom. The electron configuration is important for understanding the chemical behavior of the atom.
What is Electron Configuration?
Electron configuration is the arrangement of electrons around the nucleus of an atom. It is determined by the number of protons in the nucleus and the number of electrons in the atom. Electron configurations are usually written in terms of the orbital blocks, which are the s, p, d and f subshells. The s block is filled first, followed by the p block, then the d block and finally the f block.
What is 1s2 2s2 2p6 3s2 3p6?
The electron configuration 1s2 2s2 2p6 3s2 3p6 is the arrangement of electrons around the nucleus of an atom with 14 protons. This electron configuration is for the element Silicon (Si). Silicon has 14 protons and 14 electrons, with 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, 6 electrons in the 2p orbital, 2 electrons in the 3s orbital, and 6 electrons in the 3p orbital.
How is the Electron Configuration of Silicon Different from Other Elements?
The electron configuration of Silicon is different from other elements because it has a full 3s orbital and a half-filled 3p orbital. This is due to the fact that Silicon has 14 electrons, which is an even number. In contrast, Magnesium (Mg) has 12 electrons, which is an odd number. The electron configuration of Mg is 1s2 2s2 2p6 3s2, which has a full 2s and 2p orbital and a half-filled 3s orbital.
What is the Electron Configuration for Other Elements?
The electron configuration for other elements can vary depending on the number of protons in the nucleus. For example, Iron (Fe) has 26 protons and 26 electrons, with 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, 6 electrons in the 2p orbital, 2 electrons in the 3s orbital, 6 electrons in the 3p orbital, and 10 electrons in the 3d orbital. Thus, the electron configuration for Iron is 1s22s22p63s23p63d10.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 is the correct electronic configuration for Silicon (Si). This electron configuration is different from other elements due to the fact that Silicon has an even number of electrons, which produces a full 3s orbital and a half-filled 3p orbital. The electron configuration for other elements can vary depending on the number of protons in the nucleus.
What is the electron configuration 1s2 2s2 2p2?
Atoms are composed of electrons, protons and neutrons. Electrons are the smallest and most fundamental particles in an atom, and they occupy the outermost energy levels of the atom. To understand the arrangement of electrons around the nucleus of an atom, scientists use a notation called electron configuration.
Electron configuration is the way that electrons are arranged around the nucleus of an atom. The configuration is written using the element’s atomic number and the orbital type and subshells in which the electrons are found. The atomic number is used to determine the number of electrons in the atom, and the orbital type and subshells are used to determine how the electrons are arranged.
What is the Electron Configuration of 1s2 2s2 2p2?
The electron configuration of 1s2 2s2 2p2 is incorrect. The electron configuration of an atom depends on the atomic number of the atom. For example, the electron configuration of an atom of sodium (atomic number 11) is 1s2 2s2 2p6 3s1, and the electron configuration of an atom of magnesium (atomic number 12) is 1s2 2s2 2p6 3s2.
What is the Electron Configuration of Other Atoms?
The electron configuration of an atom of silicon (atomic number 14) is 1s2 2s2 2p6 3s2 3p2. The electron configuration of an atom of aluminum (atomic number 13) is 1s2 2s2 2p6 3s2 3p1. The electron configuration of an atom of neon (atomic number 10) is 1s2 2s2 2p6.
What is the Electron Configuration of Ions?
The electron configuration of an ion is different from the electron configuration of an atom. An ion is an atom that has gained or lost electrons, resulting in a net positive or negative charge. The electron configuration of a Fe2+ ion is 1s2 2s2 2p6 3s2 3p6 3d6, and the electron configuration of a Fe3+ ion is 1s2 2s2 2p6 3s2 3p6 3d5.
In summary, the electron configuration of 1s2 2s2 2p2 is incorrect. The electron configuration of an atom or ion depends on the atomic number of the atom. Electron configuration is a notation used to describe the arrangement of electrons around the nucleus of an atom.
What is the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7?
Understanding the electron configuration of an element is essential for understanding its chemical properties. The electron configuration of an element is a shorthand notation that describes how electrons are distributed in its atomic orbitals.
What are Electron Configurations?
Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
How the Electron Configurations are formed?
The electron configurations of elements are determined by the values of two quantum numbers, namely the principal quantum number (n) and the azimuthal quantum number (l). The principal quantum number indicates the energy level, while the azimuthal quantum number indicates the subshell. The following table shows the value of the azimuthal quantum number for each subshell.
Table of Content
- Principle Quantum Number Value
- Value of Azimuthal Quantum Number
- Resulting Subshell in the Electron Configuration
| n=1 | l=0 | 1s |
|---|---|---|
| n=2 | l=0 | 2s |
| l=1 | 2p | |
| n=3 | l=0 | 3s |
| l=1 | 3p | |
| l=2 | 3d | |
| n=4 | l=0 | 4s |
| l=1 | 4p | |
| l=2 | 4d | |
| l=3 | 4f |
Thus, it can be understood that the 1p, 2d, and 3f orbitals do not exist because the value of the azimuthal quantum number is always less than that of the principal quantum number.
Answer to the blog section title:
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is incorrect. The correct electron configuration is 1s2 2s2 2p6 3s2 3p2, which belongs to Mg (atomic number 12).
In conclusion, understanding electron configurations is essential for understanding the chemical properties of an element. The electron configuration of an element is a shorthand notation that describes how electrons are distributed in its atomic orbitals. The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is incorrect, and the correct electron configuration is 1s2 2s2 2p6 3s2 3p2, which belongs to Mg (atomic number 12).
What is the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
The electron configuration of an atom describes how electrons are distributed in its atomic orbitals. It is written with the help of subshell labels, which contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript.
The electron configuration of an atom is written in a standard notation which states all the electron-containing atomic subshells (with the number of electrons they hold written in superscript) in a sequence. The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is most easily understood by breaking it down into its component parts.
Principle Quantum Number
The principle quantum number (n) is the number associated with the energy level of an electron. It is a positive integer starting from 1. Each principal quantum number (n) has its own set of energy levels.
Value of Azimuthal Quantum Number
The value of the azimuthal quantum number (l) determines the type of subshell the electron is located in. For each principal quantum number (n), there are l values ranging from 0 to (n-1). The l value can be used to determine the name of the subshell, as shown in the table below.
| Principle Quantum Number (n) | Value of Azimuthal Quantum Number (l) | Resulting Subshell in the Electron Configuration |
|---|---|---|
| n=1 | l=0 | 1s |
| n=2 | l=0 | 2s |
| l=1 | 2p | |
| n=3 | l=0 | 3s |
| l=1 | 3p | |
| l=2 | 3d | |
| n=4 | l=0 | 4s |
| l=1 | 4p | |
| l=2 | 4d | |
| l=3 | 4f |
Thus, it can be understood that the 1p, 2d, and 3f orbitals do not exist because the value of the azimuthal quantum number is always less than that of the principal quantum number.
Therefore, the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 can be broken down into the following subshells: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, and 3d3. The 1s2 subshell is the first shell and contains two electrons, the 2s2 and 2p6 subshells are the second shell and contain four and six electrons respectively, the 3s2 and 3p6 subshells are the third shell and contain four and six electrons respectively, the 4s2 subshell is the fourth shell and contains two electrons, and the 3d3 subshell is the third shell and contains three electrons.
In conclusion, the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is the standard notation used to describe how electrons are distributed in an atom’s atomic orbitals. It is important to understand the values of the principal and azimuthal quantum numbers in order to correctly interpret the electron configuration of an atom.



Leave a Comment