Electrons are the basis of our understanding of the chemical world. They are the building blocks that organize and control the behavior of atoms and molecules. Knowing the electron configuration of an element can help us understand its chemical properties and reactivity.
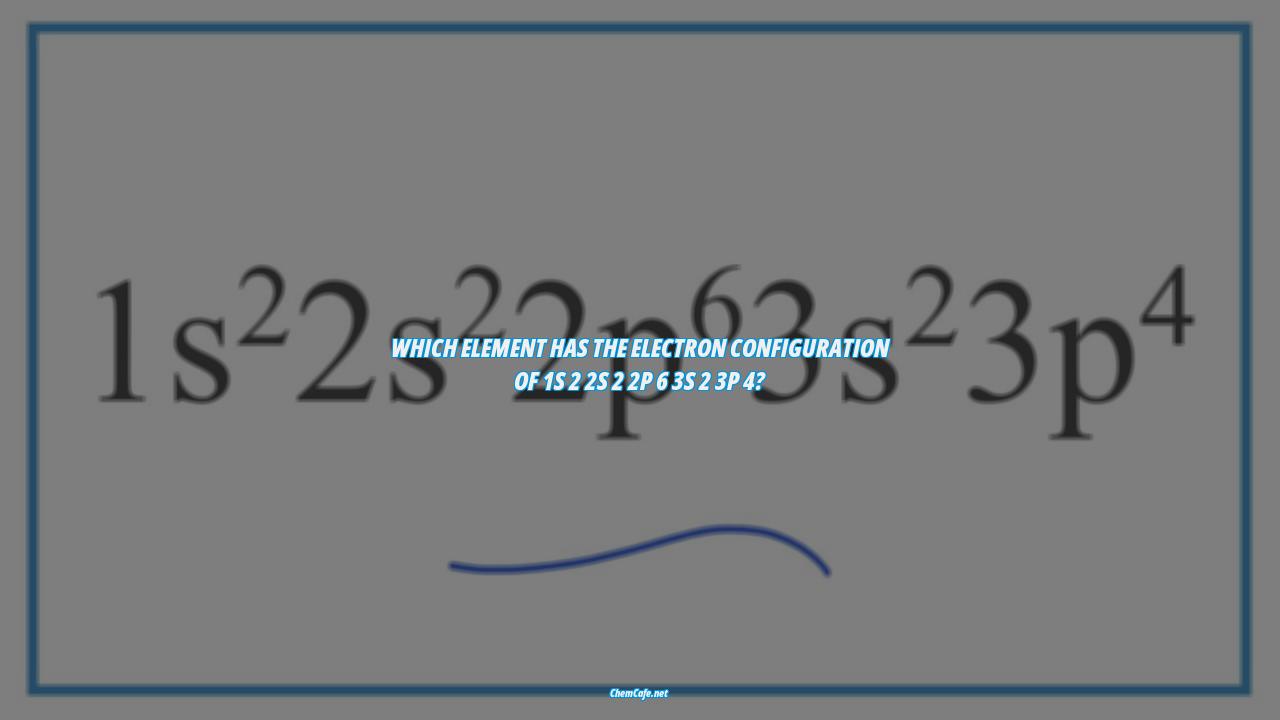
But what exactly is an electron configuration? It is a standard notation used to describe how electrons are distributed in an atom’s orbitals. Each orbital is identified by its energy level and the number of electrons it contains. These are written as superscripts in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, writing electron configurations for elements with a large atomic number can get quite lengthy. In order to simplify this process, scientists use the orbital-filling chart in Figure 5.9. This chart indicates the order in which the orbitals should be filled. For example, the electron configuration of boron is 1s22s22p6 3s2 3p4, which is derived from the orbital-filling chart.
Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. Knowing the electron configuration of an element can give us valuable insights into its properties and behavior. For example, the electron configuration of lithium is 1s22s1, which indicates that it has only one electron in its outer shell and is highly reactive.
In this article, we will explore what electron configurations are and how they are determined. We will also look at the orbital-filling chart and how it can be used to determine the electron configuration of an element. Finally, we will discuss the significance of knowing the electron configuration of an element and how it can help us understand its properties and reactivity.
Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?
Electron configurations are used to describe the arrangement of electrons in an atom. They follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
When it comes to determining the electron configuration of an element, it can be a bit tricky. But by following the orbital-filling chart in Figure 5.9, you can easily figure out the electron configuration of any element. For instance, the electron configuration of boron is:
B: 1s22s22p1
Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. The electron configurations of elements with higher atomic numbers can be written by following the orbital-filling chart in Figure 5.9.
Let’s take a look at lithium (atomic number 3), for example. We must first determine (from Figure 5.9) that the 2s sublevel is next higher in energy after the 1s sublevel. Therefore, the electron configuration of lithium is:
Li: 1s22s1
Similarly, boron (atomic number 5) has five electrons. Four electrons fill both the 1s and 2s orbitals. The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). Therefore, the electron configuration of boron is:
B: 1s22s22p1
Now let’s take a look at the electron configuration of fluorine (atomic number 9). The electron configuration of fluorine is:
F: 1s22s22p63s23p5
Now let’s look at the electron configuration of an element with an atomic number greater than 18. For example, let’s consider bromine (atomic number 35). The electron configuration of bromine is:
Br: 1s22s22p63s23p64s23d104p65s24d105p5
By following the orbital-filling chart in Figure 5.9, you can easily determine the electron configuration of any element. Now that you know the basics of electron configurations, let’s answer the question: Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?
The answer is boron (atomic number 5). Its electron configuration is:
B: 1s22s22p1
In conclusion, electron configurations are used to describe the arrangement of electrons in an atom. By following the orbital-filling chart in Figure 5.9, you can easily determine the electron configuration of any element. The electron configuration of boron is 1s 2 2s 2 2p 6 3s 2 3p 4.
Which element has electronic configuration 1 is 2 to 2 p6 3s2 3p4?
Atoms are composed of protons, neutrons, and electrons. The number of protons in an atom determines its atomic number and its identity. Electrons are found in specific shells around the nucleus of an atom, and the arrangement of these electrons is known as an electron configuration. The electron configuration for an atom can be written using a shorthand notation of the orbital types and their energy levels.
In this article, we will take a look at the electron configuration of an atom with 1s2 2s2 2p6 3s2 3p4, and learn what element this configuration corresponds to. We will also explore the properties of this element, as well as its group and atomic number.
What is the Electron Configuration of 1s2 2s2 2p6 3s2 3p4?
The electron configuration 1s2 2s2 2p6 3s2 3p4 corresponds to an element with 43 electrons. This configuration indicates that there are two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and four electrons in the 3p orbital.
Which Element Does 1s2 2s2 2p6 3s2 3p4 Correspond To?
The electron configuration 1s2 2s2 2p6 3s2 3p4 corresponds to the element Technetium (Tc). Technetium is a transition element located in group 7 in the periodic table. It has an atomic number of 43 and an atomic weight of 98.
What are the Properties of Technetium?
Technetium is a silvery-gray metal that is radioactive and highly reactive. It has the highest atomic number of all the naturally occurring elements, and it is the lightest element with no stable isotopes. Technetium has an atomic radius of 137 pm, a melting point of 2157 K, and a boiling point of 4877 K. It is a transition element, meaning that it has a partially filled d orbital in its electron configuration.
The electron configuration 1s2 2s2 2p6 3s2 3p4 corresponds to the element Technetium. Technetium is a transition element located in group 7 in the periodic table, with an atomic number of 43 and an atomic weight of 98. It is a silvery-gray metal that is radioactive and highly reactive, with an atomic radius of 137 pm, a melting point of 2157 K, and a boiling point of 4877 K.
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Electron configurations are used to describe the distribution of electrons in an atom’s orbitals. Each element has its own unique arrangement, or electron configuration, which can be used to predict its physical, chemical, and atomic properties. The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to a particular element, but what is it?
The Electron Configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 describes the distribution of electrons in an atom’s orbitals. The configuration is made up of four parts, each representing a different energy level or shell, and each shell contains a different number of orbitals. The first shell, or the 1s shell, is the closest to the nucleus and contains two electrons. The second shell, or the 2s shell, contains two electrons and the third shell, or the 2p shell, contains six electrons. The fourth shell, or the 3s shell, contains two electrons and the fifth shell, or the 3p shell, contains six electrons. The sixth shell, or the 4s shell, contains two electrons, the seventh shell, or the 3d shell, contains ten electrons, and the eighth shell, or the 4p shell, contains three electrons.
What element is the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to the element phosphorus. Phosphorus is a non-metal element in the 15th group of the periodic table and has an atomic number of 15. It is a highly reactive element and is used in fertilizers and explosives.
Notation of Electron Configurations
The electron configuration of an element is written using subshell labels. These labels consist of the shell number, the subshell name, and the number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the notation is written as ‘1s2’. The notation for the electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3.
Why are Electron Configurations Important?
Electron configurations are important for several reasons. They are used to determine the valency of an element, which is the number of electrons an atom can gain or lose in a chemical reaction. They are also used to predict the properties of a group of elements, as elements with similar electron configurations often exhibit similar properties. Finally, they are used to interpret atomic spectra, which are used to identify elements in a sample.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 belongs to the element phosphorus. This configuration is written using subshell labels and is used to describe the distribution of electrons in an atom’s orbitals. It is also used to determine the valency of an element, predict the properties of a group of elements, and interpret atomic spectra.
Which element whose atoms have these configuration 1s 2 2s 2 2p 6 3s 2 3p 5?
The electronic configuration of an element is a representation of the arrangement of electrons in the orbitals of its atoms. The configuration is expressed as a sequence of numbers, with each number representing the number of electrons in an orbital. For example, the configuration 1s2 2s2 2p6 3s2 3p5 tells us that the element has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital and five electrons in the 3p orbital.
So, the element whose atoms have these configuration 1s2 2s2 2p6 3s2 3p5 is silicon (Si). Silicon is a chemical element with atomic number 14, which means it has 14 protons and 14 electrons in its atomic structure. The electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2.
Silicon is a member of the carbon family in Group 14 of the periodic table. Elements in this group have the same electron configurations as their counterparts in the carbon family, with the principal quantum number of the outer shell increasing by 1. In the case of silicon, the outer shell has a principal quantum number of 3, instead of 2 for carbon and nitrogen.
Silicon is the second most abundant element in the Earth’s crust and is a major component of many minerals, such as quartz and opal. Silicon is also used in the production of many industrial products, such as glass and semiconductors.
In terms of its chemical properties, silicon is a non-metal and is a semiconductor. This means that it can conduct electricity under certain conditions. Silicon is also highly resistant to corrosion, making it a suitable material for many industrial applications.
Silicon is also an important component of many biological molecules. It is found in the connective tissues of plants, as well as in proteins, lipids and carbohydrates. Silicon is also found in the human body, where it is essential for the formation of bones and other tissues.
In conclusion, the element whose atoms have these configuration 1s2 2s2 2p6 3s2 3p5 is silicon. Silicon is an important element with many uses in both industry and biology. Its resistance to corrosion and its ability to conduct electricity under certain conditions make it a valuable material for many industrial applications.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
Electron configurations are a representation of the distribution of electrons in an atom. They follow a standard notation of placing all electron-containing atomic subshells in a sequence. Understanding electron configurations is essential to understanding the basic structure of an atom.
What are Electron Configurations?
Electron configurations are a way of representing the distribution of electrons in an atom. They follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). To make it easier to understand, the electron configuration can be abbreviated by eliminating subshells that contain only one electron. For example, the electron configuration of sodium can be written as [Ne]3s1.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
The given electron configuration is 1s2 2s2 2p6 3s2 3p1. The correct answer is option E. An atom of element Na (atomic number 11) contains 11 electrons. The electron configuration of this element is 1s2 2s2 2p6 3s1. Hence, this option can be neglected.
The electron configuration of Ne (atomic number 10) is 1s2 2s2 2p6. Thus, this option is incorrect. The atomic number of Al is 13, and it contains 13 electrons which are arranged as follows: 1s2 2s2 2p6 3s2 3p1. Thus, this option is the correct choice.
The electron configuration of Si (atomic number 14) is 1s2 2s2 2p6 3s2 3p2. Thus, this option is incorrect. The electron configuration of Mg (atomic number 12) is 1s2 2s2 2p6 3s2. Thus, this option is incorrect.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p1 belongs to element Al, with an atomic number of 13. Understanding electron configurations is important to understanding the basic structure of an atom, and can help in understanding chemical reactions.
What element has the electron configuration 1s 2s 2p 3s 3p?
When it comes to chemistry, it is essential to know the electron configuration of different elements. Knowing the electron configuration of an element can help you understand its chemical properties and reactivity. The electron configuration of an element is determined by the number of electrons it has in its outermost energy level. One element that has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6 is radon.
What is Radon?
Radon, symbolized as Rn, is an element in the noble gas family. It is a colorless, odorless, and tasteless gas that is naturally present in the environment. Radon is the heaviest of the noble gases and is also radioactive. Because of its radioactivity, it can be hazardous if it accumulates in enclosed spaces.
What is the Electron Configuration of Radon?
The electron configuration of radon is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6. This means that the outermost energy level of radon is filled with 7 electrons. These electrons are distributed in the s orbital, p orbital, d orbital, and f orbital of the outermost energy level.
What is the Orbital Filling Chart?
The orbital filling chart is a tool used to determine the electron configuration of an element. It is a diagram that shows the order in which electrons fill orbitals. The chart starts with the s orbital at the top and then moves down to the p orbital, d orbital, and f orbital. The electrons fill each orbital in order of increasing energy. The electrons fill the orbitals in pairs until the outermost energy level is filled with eight electrons.
What is Noble Gas Shorthand?
Noble gas shorthand is a way of writing the electron configuration of an element without writing out the entire configuration. It involves writing the symbol for the noble gas before the element symbol and then adding the extra information. For example, the electron configuration of radon can be written as [Rn] 5f14 6d10 7s2 7p6. This shorthand can be used to save room when writing out electron configurations.
In conclusion, radon is an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6. This configuration can be written in noble gas shorthand as [Rn] 5f14 6d10 7s2 7p6. Knowing the electron configuration of an element can help you understand its chemical properties and reactivity. The orbital filling chart is a useful tool for writing the electron configuration of an element.



Leave a Comment