Atomic mass is a fundamental concept in the study of Chemistry and Physics. It is the amount of mass of an element or a group of atoms that can be attributed to the protons, neutrons, and electrons it contains. This value is important to understand the properties of elements and how they interact with each other. It is also essential in determining the elements’ atomic weight.
Atomic mass is calculated in a few different ways. The most common way is to refer to the periodic table and use the atomic number of the element in question. This method is the most accurate, as it takes into account the mass of all the protons and neutrons present in the atom. It gives an accurate representation of the element’s atomic weight.
Another way to calculate atomic mass is to add up the masses of all the protons and neutrons present in the nucleus of the atom. This gives a more simplified calculation but still gives an accurate representation of the element’s atomic weight.
Finally, the mass of a single atom can be found by taking the average mass of a group of atoms. This method is not as accurate as the other two, but still gives a good indication of the element’s atomic weight.
Overall, understanding atomic mass is important for anyone studying Chemistry or Physics. It has a variety of uses, from understanding the properties of elements to determining the elements’ atomic weight. Knowing how to calculate atomic mass is essential in order to accurately measure this important value.
What is atomic mass and how is it calculated?
Atomic mass is an important concept in chemistry and physics, and is used to measure the amount of matter in an atom. The atomic mass of an atom is the sum of the masses of the protons, neutrons, and electrons in that atom, and is typically represented by the symbol A. Atomic mass can be calculated in several ways, depending on the information that is available.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. This is typically represented by the symbol A, and is measured in atomic mass units (amu). The mass of a single atom of carbon, for example, is 12 amu. The mass of a single atom of hydrogen is 1 amu.
How Is Atomic Mass Calculated?
There are three different methods to calculate the atomic mass of an atom:
By Referring to the Periodic Table
In the periodic table, the atomic number of an element is typically indicated under its symbol. In general, however, the atomic mass of an atom will be very similar to its mass number, although the decimal places will have some variation.
By Adding the Masses of the Protons and Neutrons
In order to calculate the mass of a single atom of an element, one can consider using the number of protons and neutrons present in its nucleus. By adding the masses of each proton and neutron present in the nucleus of a given atom, one can extract the atomic mass of that atom. In the general terminology, atomic mass can also be defined as the number of the protons and neutrons.
By Calculating the Average Mass of an Element
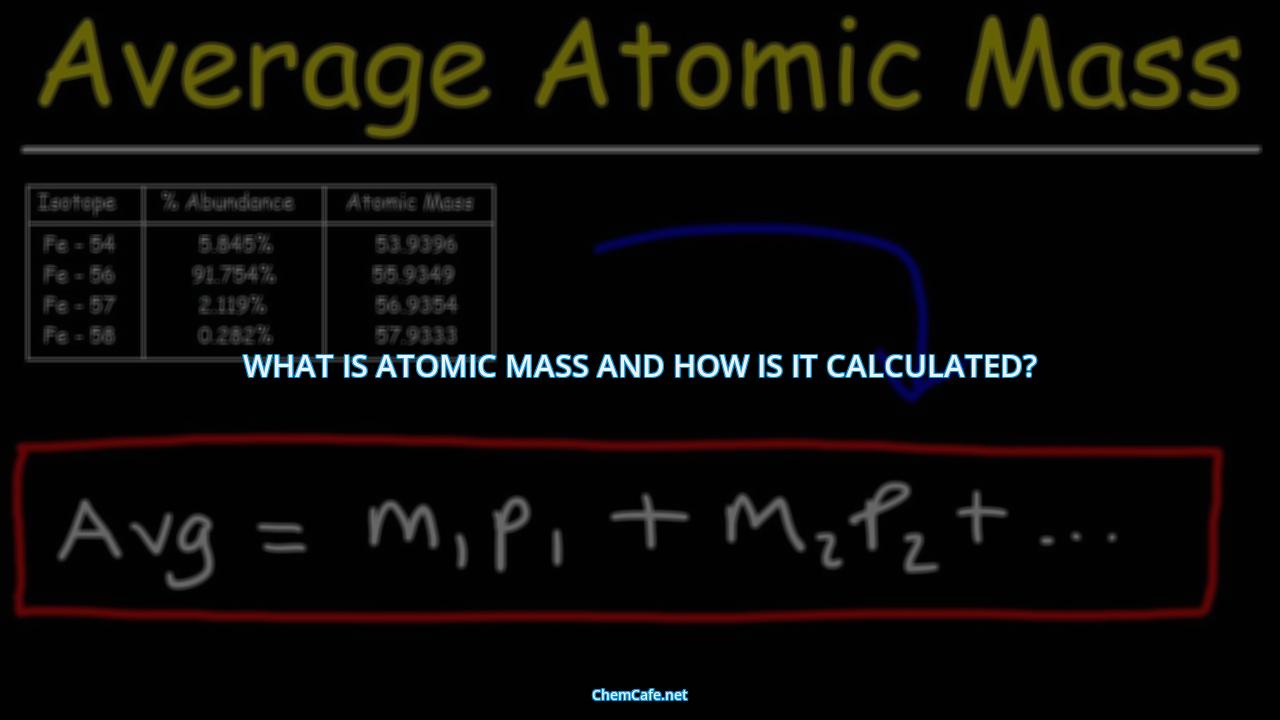
The average mass of an element can be calculated by taking the weighted average of the mass of each of its isotopes. This method is especially useful when dealing with elements that have multiple isotopes.
Atomic mass is an important concept in physics and chemistry, and is used to measure the amount of matter in an atom. It can be calculated in several ways, depending on the information available. By referring to the periodic table, adding the masses of the protons and neutrons, or calculating the average mass of an element, one can determine the atomic mass of an atom.
How do you find the mass of an atom?
Atoms are the foundation of all matter and understanding the mass of an atom is essential to understanding the physical and chemical properties of a substance. Mass is a measure of how much matter is in an atom, and it is typically expressed in atomic mass units (amu). But how do you find the mass of an atom?
What is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The mass of an atom is a weighted average that is largely determined by the number of its protons and neutrons, whereas the number of protons and electrons determines its charge. Each atom of an element contains the same number of protons, known as the atomic number (Z).
3 Ways to Find Atomic Mass
The method used to find atomic mass depends on whether you’re looking at a single atom, a natural sample, or a sample containing a known ratio of isotopes:
1) Look Up Atomic Mass on the Periodic Table
If it’s your first encounter with chemistry, your instructor will want you to learn how to use the periodic table to find the atomic mass (atomic weight) of an element. You may be asked to calculate atomic mass in chemistry or physics. There is more than one way to find atomic mass. Which method you use depends on the information you’re given.
2) Calculate the Mass of a Single Atom
If you know the number of protons, neutrons, and electrons in an atom, you can calculate the mass of that single atom. You can calculate the mass of one atom by adding up the masses of its protons, neutrons, and electrons. To calculate the mass of an atom, you need to know the mass of each of its components. The mass of a proton is 1.0073 amu, the mass of a neutron is 1.0087 amu, and the mass of an electron is 0.0005 amu.
3) Calculate the Atomic Weight of an Element
If you have a natural sample of an element or a sample containing a known ratio of isotopes, you can calculate the average atomic weight of the element. This is done by calculating the weighted average of the masses of the different isotopes of an element. For example, if you have a sample containing two isotopes of carbon, 12C and 13C, you can calculate the average atomic weight of the sample by multiplying the mass of each isotope by its abundance and adding the two values together.
The answer to the question of how do you find the mass of an atom is that it depends on the information you have. If you know the number of protons, neutrons, and electrons in an atom, you can calculate the mass of that single atom. If you have a natural sample of an element or a sample containing a known ratio of isotopes, you can calculate the average atomic weight of the element. Finally, you can look up the atomic mass of an element on the periodic table.
Regardless of the method you use, understanding the mass of an atom is essential to understanding the physical and chemical properties of a substance. Knowing the mass of an atom can help you determine the isotopic composition of a sample, which is important for understanding the chemistry of a substance.
How is atomic mass calculated?
Atomic mass is a key concept in chemistry and physics and is used to identify the elements and measure the mass of single atoms. But how is atomic mass calculated? The answer depends on the information you have and the method you use. Here, we’ll explain the basics and provide an overview of the main methods for calculating atomic mass.
What is atomic mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass of a group of atoms. It is measured in atomic mass units (amu). This is a relative unit of mass, where an atom of carbon-12 is assigned a mass of 12 amu.
How to calculate atomic mass
There are a few different methods for calculating atomic mass, and the method you choose depends on the information you have.
Addition of protons and neutrons
One of the simplest ways to calculate atomic mass is to add up the masses of the protons and neutrons in the nucleus of the atom. This is also known as the mass number. To do this, you need to know the number of protons and neutrons in the nucleus, which is equal to the atomic number.
Reference to the periodic table
You can also use the periodic table to calculate atomic mass. The atomic number is typically indicated underneath the representation of the element. The atomic mass is usually close to the mass number, but with a few decimal places of variation.
Using isotopic composition
If you know the isotopic composition of an element, you can use this to calculate the atomic mass. Isotopic composition refers to the relative abundance of different isotopes of an element in a sample. You can use the mass number and abundance of each isotope to calculate the average atomic mass.
Using mass spectrometry
Finally, you can also use mass spectrometry to determine the atomic mass. Mass spectrometry is a technique used to measure the mass of a sample. It works by breaking up the sample into its component parts, which are then detected and measured. The mass of the atom can then be calculated from the mass of each component.
In summary, there are several different methods for calculating atomic mass. The method you choose depends on the information you have. The most common methods are adding up the masses of the protons and neutrons, referencing the periodic table, using isotopic composition, and using mass spectrometry.
What is atomic mass and how do you calculate it?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. It is also referred to as atomic weight. Understanding atomic mass is essential for studying chemistry and physics. It is also helpful to know how to calculate it, as it can be used to determine the mass of a given element or molecule.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass is the mass of an atomic particle or molecule. With this system, the masses of a proton and a neutron are nearly equal to 1 atomic mass unit, while the electron has a mass close to 0.0005 atomic mass unit.
Atomic mass is also called atomic weight. For atoms, the protons and neutrons of the nucleus account for almost all of the mass, and the atomic mass measured in u has nearly the same value as the mass number. Masses of fundamental atomic particles are often expressed in atomic mass units (u). “One atomic mass unit (1u) is one twelfth of the mass of an atom of carbon with six protons and six neutrons.”
How to Calculate Atomic Mass?
There are several ways to calculate atomic mass. The most common methods are based on the mass of a single atom, or the average mass of a group of atoms.
Calculate the Mass of a Single Atom
When calculating the mass of a single atom, you must first determine the number of protons, neutrons, and electrons that make up the atom. Then, you can use the atomic mass unit (u) to calculate the atom’s mass. The atomic mass unit is equal to the mass of a proton or neutron, which is 1.0073 and 1.0087 respectively. To calculate the atomic mass of a single atom, simply multiply the number of protons and neutrons by the atomic mass unit.
Calculate the Average Mass of a Group of Atoms
To calculate the average mass of a group of atoms, you must first determine the average number of protons, neutrons, and electrons in the group. This can be done by dividing the total number of protons, neutrons, and electrons in the group by the number of atoms in the group. Then, you can use the atomic mass unit to calculate the average mass of the group.
Atomic mass is an important concept in chemistry and physics. It is defined as the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. There are several ways to calculate atomic mass, depending on the information you are given. Knowing how to calculate atomic mass can be a helpful tool when studying chemistry and physics.
How to calculate atomic mass?
Atomic mass is an important concept when studying chemistry or physics. Knowing how to calculate atomic mass can help you understand the properties of different elements and their compounds.
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. This can be calculated in several ways, depending on the information you have available.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. This is usually expressed in atomic mass units (amu).
Adding Protons and Neutrons
One way to calculate the mass of a single atom of an element is to consider the number of protons and neutrons present in its nucleus. By adding the masses of each proton and neutron present in the nucleus of a given atom, you can calculate the atomic mass of that atom. In more general terms, the atomic mass can be defined as the sum of the protons and neutrons.
Calculating Atomic Mass
There are three different methods to calculate the atomic mass.
Using the Periodic Table
In the periodic table, an atomic number is typically indicated beneath the representation of an element. This number is equal to the number of protons in the nucleus of the atom. The atomic mass is usually very close to the mass number (the sum of the protons and neutrons in the nucleus), although there may be some variation in the decimal places.
Using the Molar Mass
The molar mass of an element is the mass of one mole of that element. A mole is defined as 6.022 x 10^23 atoms. In order to calculate the molar mass of an element, you need to know its atomic mass. This can be found in the periodic table. Once you have the molar mass, you can calculate the atomic mass of the element by dividing the molar mass by 6.022 x 10^23.
Using Isotope Abundance
If you know the isotope abundance of an element, you can calculate the atomic mass by multiplying the mass of each isotope by its abundance and then adding these values together. For example, if an element has an isotope with a mass of 24 and an abundance of 0.7, and another with a mass of 26 and an abundance of 0.3, then the atomic mass would be calculated as 24 x 0.7 + 26 x 0.3 = 24.6.
As you can see, there are several ways to calculate atomic mass, depending on the information you have available. It’s important to understand the concept of atomic mass, as well as the different methods of calculating it, in order to accurately study the properties of different elements.
What is atomic mass in easy way?
Atoms are the basic building blocks of matter. They are the smallest components of a chemical element, which is a substance that cannot be broken down into simpler material. Atoms have specific properties that will determine their chemical and physical nature. One of these properties is their atomic mass. This article will discuss atomic mass and its role in chemistry.
What is Atomic Mass?
Atomic mass is a measure of the total mass of all the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Atoms have three basic components: protons (positively charged particles), neutrons (non-charged particles), and electrons (negatively charged particles). Protons and neutrons are found in the nucleus, which is the core of the atom.
Atomic mass is typically calculated by adding the number of protons and neutrons together, ignoring the electrons because of their small size. Daltons are the standard units used for measuring atomic mass. One Dalton is equal to one-twelfth the mass of a single carbon-12 atom. The atomic mass of an element is the weighted average of the atomic masses of its isotopes.
How to Calculate Atomic Mass?
There are two main ways to calculate an atom’s atomic mass. The first is to add together the number of protons and neutrons in an atom. This is the most common method used when dealing with single atoms. The second method is to find the weighted average of the atomic masses of its isotopes. This is typically used when dealing with a group of atoms.
The atomic mass of an element can also be found on the periodic table. Every element has a unique atomic mass, which is listed in the periodic table. This can be used as a reference to find the atomic mass of an element quickly.
Atomic Mass and Its Role in Chemistry
Atomic mass is important in chemistry because it helps to determine the properties of atoms and molecules. Different elements have different atomic masses, and the properties of a substance will depend on the atomic mass of the element it is made up of. The atomic mass of an element also helps to determine the amount of energy released or absorbed in chemical reactions.
Atomic mass is also important in physics as it helps us to understand the structure of the universe. The mass of an atom affects its gravitational force, which is important in the study of astrophysics. Atomic mass is also important in nuclear physics, where it is used to determine the amount of energy released in nuclear reactions.
Atomic mass is an important property of atoms and molecules. It is equal to the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Atomic mass is typically calculated by adding the number of protons and neutrons together, ignoring the electrons because of their small size. Atomic mass is important in chemistry because it helps to determine the properties of atoms and molecules, and in physics because it helps us to understand the structure of the universe.




Leave a Comment