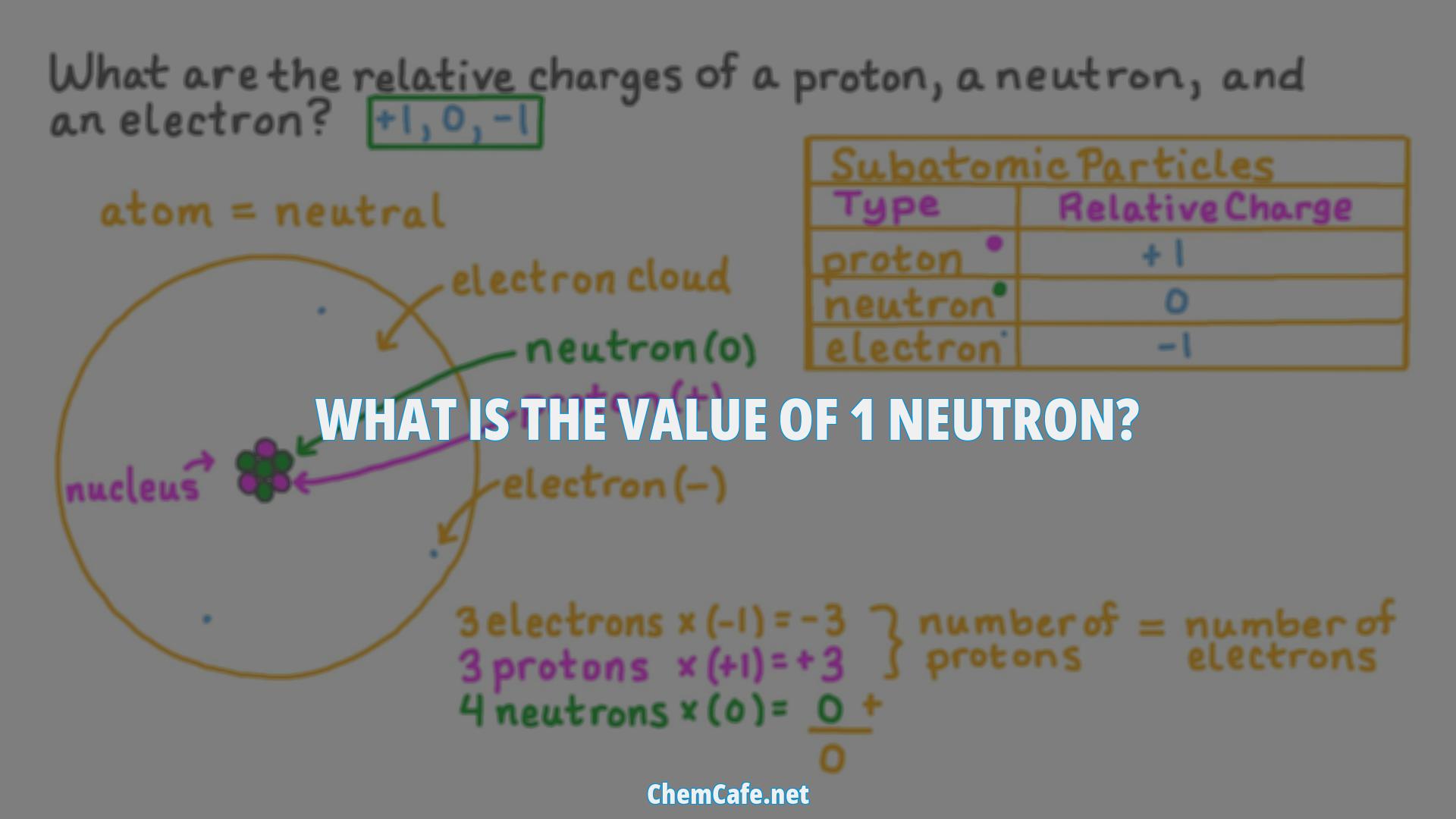
Neutrons are subatomic particles with no electrical charge, and they are the building blocks of atomic nuclei. Found in the nucleus of atoms, together with protons, neutrons have a mass of 940.6 MeV/c² or approximately 1.6749 × 10−27 kg on the atomic mass unit (u) scale. They are formed through fusion in the core of stars and can interact with other particles in a variety of ways. So, what is the value of 1 neutron?
In order to understand the value of 1 neutron, it is important to look at the concept of mass. Mass is a measure of an object’s resistance to being accelerated by a force, and the mass of a neutron is slightly greater in magnitude than that of a proton. In fact, the mass of a free neutron is 1.6749286 x 10⁻²⁷ kg or 939,565,346 eV/c². This mass can also be approximated to one atomic mass unit (amu) and is referred to as the rest mass of the neutron.
In addition to its mass, the neutron has a charge associated with it. Neutrons, along with protons, are collectively referred to as nucleons since they behave in a similar manner inside the nuclei of atoms. The overall nuclear and chemical properties of an element are usually determined by the total number of protons in its atomic nucleus (atomic number) and the total number of neutrons in its atomic nucleus (usually referred to as the neutron number).
The value of 1 neutron is therefore determined by its mass and charge. In terms of mass, 1 neutron has a mass of 1.6749286 x 10⁻²⁷ kg or 939,565,346 eV/c², which can be approximated to one atomic mass unit (amu). In terms of charge, neutrons have no net electric charge associated with them.
To summarize, the value of 1 neutron is determined by its mass and charge. In terms of mass, 1 neutron has a mass of 1.6749286 x 10⁻²⁷ kg or 939,565,346 eV/c², which can be approximated to one atomic mass unit (amu). In terms of charge, neutrons have no net electric charge associated with them.
What is the value of 1 neutron?
Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei. They are usually denoted by the symbol n or no and have no net electric charge associated with them. They have a mass that is slightly greater than that of a proton, and they are collectively referred to as nucleons. In this article, we will discuss the value of 1 neutron, including its mass and relative mass.
What are Neutrons?
Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei. They are usually denoted by the symbol n or no. Neutrons do not have any net electric charge associated with them. They do, however, have a mass which is slightly greater in magnitude than that of a proton. Neutrons and protons are collectively referred to as nucleons since they behave in a similar manner inside the nuclei of atoms.
The mass of a neutron can be roughly approximated to one atomic mass unit (often abbreviated to amu). The branch of science that deals with the study of the properties of neutrons and the interactions of these subatomic particles with other matter and electromagnetic radiation are called nuclear physics. The overall nuclear and chemical properties of an element are usually determined by the total number of protons in its atomic nucleus (atomic number) and the total number of neutrons in its atomic nucleus (usually referred to as the neutron number). The sum of the total number of protons in an atomic nucleus and the total number of neutrons in the atomic nucleus yields the mass number of that atomic nucleus.
Mass of One Neutron
The mass of a free neutron is 1.6749286 x 10⁻²⁷ kg or 939,565,346 eV/c². In common particle physics, units of mass and energy are interchangeable. Here, eV stands for electron-volt which is equivalent to 1.6 x 10⁻¹⁹ J. c = speed of light = 3 x 10⁸ m/s. Since 1 kg = 5.6095883571872E+35 eV, so 1.6749286 x 10⁻²⁷kg = (5.6095883571872E+35) x (1.6749286 x 10⁻²⁷). Mass of one neutron, mn = 939,565,413.3 eV. Thus, regarding mega-electron volt, 1 MeV = 10,00,000 eV and mass of neutron, mn = 939.565346 MeV/c².
Relative Mass of Neutron
An atom contains three subatomic particles, namely proton, electron, and neutron. The proton and neutron are found inside the nucleus at the centre of an atom. The nucleus is smaller than the size of an atom as a whole, and the electrons are arranged in shells around them. The mass of a proton is 1.6726231 x 10⁻²⁷ kg whereas the mass of the neutron, mn = 1.6726231 x 10⁻²⁷ kg.
Mass of Neutron in Grams
We know the mass of neutron in kg is 1.6726231 x 10⁻²⁷ Kg. We also know that 1 kg = 10³ g. So, mass of neutron in grams = 1.6726231 x 10⁻²⁷ x 10³ mn= 1.6726231 x 10⁻²⁴ g.
Mass of Neutron in Amu
The rest mass of the neutron that we have calculated above is in the unit of kg. In amu or atomic mass unit, the mass of neutron is calculated as, since 1 kg = 6.0229552894949E+26 amu. So, 1.6749286 x 10⁻²⁷kg = 1.6749286 x 10⁻²⁷ x 6.0229552894949E + 26. Mass of neutron, mn (amu) = 1.008664904(14) amu.
Rest Mass of Neutron
The concept of rest mass is very simple. We generally think of mass as being a constant quantity for an object. However, the theory of relativity tells us that energy and mass are interchangeable. It means that the mass of a body increases with the increase in its velocity relative to the observer. Energy gets affected by increasing an object’s mass. So, the minimum mass of an object is when it is stationary.
In conclusion, the value of 1 neutron is 1.008664904(14) amu or 939.565346 MeV/c². Neutrons are essential components of atomic nuclei and are important for understanding nuclear physics and chemistry. This article has discussed the value of 1 neutron, its mass and relative mass, and the concept of rest mass in detail.
Is a neutrons mass 1?
Neutrons are subatomic particles with no electrical charge, making them neutral in nature. They are found in the nucleus, or center, of atoms, along with protons. The mass of a neutron is slightly greater than that of a proton, and is often approximated to one atomic mass unit (amu). But, what is the exact mass of a neutron?
Mass of Neutron in Grams
The mass of a neutron in kilograms is 1.6726231 x 10⁻²⁷ Kg. To find the mass of a neutron in grams, we simply multiply this figure by 10³. This results in 1.6726231 x 10⁻²⁴ g.
Mass of Neutron in Amu
We can also express the mass of a neutron in atomic mass units (amu). Since 1 kg = 6.0229552894949E+26 amu, we can calculate the mass of a neutron in amu as 1.6749286 x 10⁻²⁷ x 6.0229552894949E + 26. This yields a result of 1.008664904(14) amu.
Rest Mass of Neutron
The rest mass of an object is the minimum mass that it can have, and is measured when the object is stationary. Since the theory of relativity tells us that energy and mass are interchangeable, the mass of a body increases with the increase in its velocity relative to the observer.
Charge and Mass of a Neutron
A neutron has no electric charge associated with it, making it a subatomic particle with neutral charge. Its mass is equivalent to 1.008 atomic mass units. The estimated mass of a neutron in kilograms is 1.674 * 10-27 kg. Since neutrons have no electric field, they cannot be measured using analytical methods of mass spectrometry.
To measure the mass of a neutron, we can use the deuterium nucleus, which consists of one proton, one neutron, and one electron. By deducting the mass of a proton from the mass of a deuterium atom, we can determine the mass of the neutron. This is because the mass of an electron is very small compared to the masses of a proton and a neutron.
In conclusion, the mass of a neutron is 1.674927471(21) * 10-27 kg. This can be determined using various deceptive techniques, such as measuring the mass of a deuterium nucleus and deducting the mass of a proton. Neutrons have no electrical charge, making them subatomic particles with neutral charge. They are found in the nucleus of atoms, along with protons. The mass of a neutron is slightly greater than that of a proton, and is often approximated to one atomic mass unit (amu).
Is the mass of a neutron 1 or 0?
The mass of a neutron is neither 1 nor 0. It is an important subatomic particle found in the nucleus of atoms, and it has a mass of 1.008 atomic mass units (amu). This mass is equivalent to 1.674 x 10-27 kg, which is very small in comparison to the mass of a proton, which is 1.6726231 x 10-27 kg.
Charge and Mass of a Neutron
A neutron has no electric charge associated with it. As a result, neutrons are subatomic particles with neutral charges. A neutron has a mass equivalent to 1.008 atomic mass units. The estimated mass of a neutron can be 1.674 * 10-27 kg when expressed in kilograms. The analytical method of mass spectrometry cannot easily measure the mass of neutrons since they have no electric field.
Deuterium
Deuterium, a hydrogen isotope, has one proton, one neutron, and one electron as part of its atomic structure. The mass of a deuterium nucleus can be calculated by deducting the mass of a proton from that of a deuterium nucleus. Because the electron’s mass is extremely small compared to the masses of the neutron and proton, the mass of a neutron can be estimated by deducting the mass of a proton from the mass of a deuterium atom.
Rest Mass of Neutron
The concept of rest mass is very simple. We generally think of mass as being a constant quantity for an object. However, the theory of relativity tells us that energy and mass are interchangeable. It means that the mass of a body increases with the increase in its velocity relative to the observer. Energy gets affected by increasing an object’s mass. So, the minimum mass of an object is when it is stationary.
Mass of Neutron in Grams
We know the mass of neutron in kg is 1.6726231 x 10-27 Kg.We also know that 1 kg = 10³ g. So, mass of neutron in grams = 1.6726231 x 10-27 x 10³. mn= 1.6726231 x 10-24 g.
Mass of Neutron in Amu
The rest mass of the neutron that we have calculated above is in the unit of kg.In amu or atomic mass unit, the mass of neutron is calculated as. Since 1 kg = 6.0229552894949E+26 amu, 1.6749286 x 10-27kg = 1.6749286 x 10-27 x 6.0229552894949E + 26. mn (amu) = 1.008664904(14) amu.
What is the Mass of a Neutron?
Considering that electrons are only approximately 0.054 percent as massive as neutrons and protons are around 99.86 percent as massive. Each atom’s relative mass is expressed in kilograms. Therefore, the relative mass of a neutron is 1. Mass Neutron (mN) = 1.674927471(21) * 10-27 kg.
What are Neutrons?
Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei. They are usually denoted by the symbol n or no. Neutrons do not have any net electric charge associated with them. They do, however, have a mass which is slightly greater in magnitude than that of a proton. Neutrons and protons are collectively referred to as nucleons since they behave in a similar manner inside the nuclei of atoms. The mass of a neutron can be roughly approximated to one atomic mass unit (often abbreviated to amu). The branch of science that deals with the study of the properties of neutrons and the interactions of these subatomic particles with other matter and electromagnetic radiation are called nuclear physics.
The overall nuclear and chemical properties of an element are usually determined by the total number of protons in its atomic nucleus (atomic number) and the total number of neutrons in its atomic nucleus (usually referred to as the neutron number). The sum of the total number of protons in an atomic nucleus and the total number of neutrons in the atomic nucleus yields the mass number of that atomic nucleus.
In conclusion, the mass of a neutron is neither 1 nor 0, but rather 1.008 atomic mass units (amu). This mass is equivalent to 1.674 x 10-27 kg, which is very small in comparison to the mass of a proton, which is 1.6726231 x 10-27 kg. Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei, and they have a mass which is slightly greater in magnitude than that of a proton. The mass of a neutron can be roughly approximated to one atomic mass unit (often abbreviated to amu) and can be calculated from the mass of a deuterium atom by deducting the mass of a proton.
Is a neutrons mass 1 Dalton?
Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei. They are usually denoted by the symbol n or no, and do not have any net electric charge associated with them. Neutrons and protons are collectively referred to as nucleons since they behave in a similar manner inside the nuclei of atoms.
The mass of a neutron can be roughly approximated to one atomic mass unit (often abbreviated to amu). This unit of mass is also known as the unified atomic mass unit (u) or dalton (Da). The branch of science that deals with the study of the properties of neutrons and the interactions of these subatomic particles with other matter and electromagnetic radiation are called nuclear physics.
What is the Dalton?
The dalton (Da) is a small unit of mass used to express atomic and molecular masses. It is defined to be one twelfth of the mass of an unbound atom of the carbon-12 nuclide, at rest and in its ground state. This means that 1 u = 1/NA gram = 1/ (1000 NA) kg (where NA is Avogadro’s number). This can be expressed as 1 u ≈ 1.660538782(83) × 10−27 kg ≈ 931.494028(23) MeV/c2.
How to Convert Neutron Mass to Dalton?
To convert neutron mass to dalton, use the following conversion table:
Neutron Mass to Dalton Conversion Table
| Neutron Mass | Dalton |
| 0.01 Neutron mass | 0.0100867109 dalton |
| 0.1 Neutron mass | 0.1008671087 dalton |
| 1 Neutron mass | 1.0086710869 dalton |
| 2 Neutron mass | 2.0173421739 dalton |
| 3 Neutron mass | 3.0260132608 dalton |
| 5 Neutron mass | 5.0433554347 dalton |
| 10 Neutron mass | 10.0867108694 dalton |
| 20 Neutron mass | 20.1734217388 dalton |
| 50 Neutron mass | 50.4335543471 dalton |
| 100 Neutron mass | 100.8671086941 dalton |
| 1000 Neutron mass | 1008.6710869413 dalton |
To convert neutron mass to dalton, simply multiply the neutron mass by 1.0086710869. For example, to convert 15 Neutron mass to dalton, multiply 15 by 1.0086710869, the answer is 15.1300663041 dalton.
What are Neutrons?
The overall nuclear and chemical properties of an element are usually determined by the total number of protons in its atomic nucleus (atomic number) and the total number of neutrons in its atomic nucleus (usually referred to as the neutron number). The sum of the total number of protons in an atomic nucleus and the total number of neutrons in the atomic nucleus yields the mass number of that atomic nucleus.
Neutrons are subatomic particles that are one of the primary constituents of atomic nuclei. They are usually denoted by the symbol n or no and do not have any net electric charge associated with them. Neutrons and protons are collectively referred to as nucleons since they behave in a similar manner inside the nuclei of atoms. The mass of a neutron can be roughly approximated to one atomic mass unit (often abbreviated to amu).
Additional recommended knowledge
The unified atomic mass unit (u), or dalton (Da), is a small unit of mass used to express atomic and molecular masses. It is defined to be one twelfth of the mass of an unbound atom of the carbon-12 nuclide, at rest and in its ground state. It appears that your browser has JavaScript disabled. Rosalind requires your browser to be JavaScript enabled.
Atomic masses are often written without any unit and then the unified atomic mass unit is implied. In biochemistry and molecular biology literature (particularly in reference to proteins), the term “dalton” is used, with the symbol Da.
A list of objects which have a mass of about 1 u can be seen in 1 E-27 kg. The symbol amu for atomic mass unit is not a symbol for the unified atomic mass unit. Its use is an historical artifact (written during the time when the amu scales were used), an error (possibly deriving from confusion about historical usage), or correctly referring to the historical scales that used it (see History).
The dalton (Da) is a small unit of mass used to express atomic and molecular masses. It is defined to be one twelfth of the mass of an unbound atom of the carbon-12 nuclide, at rest and in its ground state. This means that 1 u = 1/NA gram = 1/ (1000 NA) kg (where NA is Avogadro’s number). This can be expressed as 1 u ≈ 1.660538782(83) × 10−27 kg ≈ 931.494028(23) MeV/c2.
The dalton is only suitable for measuring the mass of objects on the molecular scale. 1 gram is equivalent to $6.02 times 10^{23}$ daltons. Accordingly, the Dalton is only suitable for measuring the mass of objects on the molecular scale.
In conclusion, the answer to the question “Is a neutron’s mass 1 dalton?” is no. The mass of a neutron is approximately equal to one atomic mass unit (amu), which is also known as the unified atomic mass unit (u) or dalton (Da). To convert neutron mass to dalton, multiply the neutron mass by 1.0086710869.
What is the charge of the neutron?
The neutron is an electrically neutral subatomic particle that forms the nucleus of an atom. It is composed of two down quarks, each with 1/3 elementary charge, and one up quark, with 2/3 elementary charge. This means that the neutron has a neutral charge, neither positive nor negative.
Understanding the Charge of the Neutron
The charge of the neutron is believed to be from the charge of the quarks that make up the nucleons (protons and neutrons). A proton is made of two Up quarks, with 2/3 positive charge each and one Down Quark with a negative 1/3 charge (2/3 + 2/3 + -1/3 = 1). A neutron is made up of two Down quarks with a negative 1/3 charge each and one Up quark with a positive 2/3 charge. (-1/3 + -1/3 + 2/3 = 0). This means that the neutron has a neutral charge, neither positive nor negative.
History of the Charge of the Neutron
The notion of the neutron having a neutral charge was first put forth in 1947 by Enrico Fermi, a Nobel laureate noted for his role in developing the first nuclear reactor. However, new research by a University of Washington physicist, Gerald A. Miller, has found that the neutron has a negative charge both in its inner core and its outer edge, with a positive charge sandwiched in between to make the particle electrically neutral.
Implications of the Charge of the Neutron
This discovery changes scientific understanding of how neutrons interact with negatively charged electrons and positively charged protons. It is significant because it is a clear fact of nature that we didn’t know before. Now we know it. This knowledge can help scientists better understand the behavior of particles in the nucleus of an atom and how they interact with other particles in the universe.
In conclusion, the neutron has a neutral charge, neither positive nor negative. This charge is believed to be from the charge of the quarks that make up the nucleons (protons and neutrons). The notion of the neutron having a neutral charge was first put forth in 1947 by Enrico Fermi, and recent research has confirmed this hypothesis. This discovery changes scientific understanding of how neutrons interact with negatively charged electrons and positively charged protons, and can help scientists better understand the behavior of particles in the nucleus of an atom and how they interact with other particles in the universe.
Is a neutron equal to 1 amu?
When it comes to the study of atomic structure and the properties of atoms and their constituents, the concept of atomic mass is of utmost importance. Atomic mass is the mass of an atom, which is determined by the number of protons and neutrons it contains. The atomic mass unit (amu) is used to represent the mass of an individual atom, and it is equal to 1.6605 x 10-24 grams. But is a neutron equal to 1 amu? Let’s find out.
What is an Atomic Mass Unit (amu)?
An atomic mass unit (amu) is a unit of measurement used to represent the mass of an individual atom. It is equal to 1.6605 x 10-24 grams, which is approximately equal to the mass of a single proton or neutron. This unit of measurement is commonly used in the field of nuclear physics, where it is important to accurately measure the mass of individual atoms.
What is a Neutron?
A neutron is an electrically neutral subatomic particle that is found in the nucleus of an atom. It has a mass of 1.67492749804 x 10-27 kg, which is slightly greater than that of the proton, but 1,838.68 times greater than that of the electron. Neutrons, along with protons, are part of the nucleus of an atom, and they account for 99.9 percent of the atom’s mass.
What is the Difference Between a Neutron and Atomic Mass Unit (amu)?
The main difference between a neutron and an atomic mass unit (amu) is that a neutron is a subatomic particle that has a mass of 1.67492749804 x 10-27 kg, whereas an atomic mass unit (amu) is a unit of measurement used to represent the mass of an individual atom, and it is equal to 1.6605 x 10-24 grams.
Is a Neutron Equal to 1 amu?
No, a neutron is not equal to 1 amu. The mass of a neutron is 1.67492749804 x 10-27 kg, which is slightly greater than that of the proton, but 1,838.68 times greater than that of the electron. On the other hand, the mass of an atomic mass unit (amu) is equal to 1.6605 x 10-24 grams, which is approximately equal to the mass of a single proton or neutron.
What is the Relationship Between a Neutron and Atomic Mass Unit (amu)?
The mass of a neutron is slightly greater than that of a proton, but 1,838.68 times greater than that of the electron. The mass of an atomic mass unit (amu) is equal to 1.6605 x 10-24 grams, which is approximately equal to the mass of a single proton or neutron. Therefore, while a neutron is not equal to 1 amu, the mass of a neutron is related to the mass of an atomic mass unit (amu).
What is the Molar Mass of a Hydrogen Atom?
The molar mass of a hydrogen atom is equal to 1 g/mol. This is because the mass of a hydrogen atom is 1 a.m.u., and 1.6726219 x 10-24 grams x 6.022 x 1023 = 1.007 g/mol, which is close to the mass that you find in a periodic table.
What is the Relationship Between Isotopes and Atomic Mass?
Isotopes are various forms of an element that have the same number of protons, but a different number of neutrons. Scientists determine the atomic mass by calculating the mean of the mass numbers for its naturally-occurring isotopes. Therefore, the relationship between isotopes and atomic mass is that isotopes are used to calculate the atomic mass of an element.
In conclusion, the mass of a neutron is 1.67492749804 x 10-27 kg, which is slightly greater than that of the proton, but 1,838.68 times greater than that of the electron. On the other hand, the mass of an atomic mass unit (amu) is equal to 1.6605 x 10-24 grams, which is approximately equal to the mass of a single proton or neutron. Therefore, while a neutron is not equal to 1 amu, the mass of a neutron is related to the mass of an atomic mass unit (amu). Isotopes are used to calculate the atomic mass of an element.
Is the mass of a proton 1 dalton?
The answer is yes, the mass of a proton is approximately 1 dalton. This is because a dalton is defined as 1/12 the mass of a single atom of carbon-12, and a proton has approximately the same mass as a carbon-12 atom.
A proton is one of three main particles that make up the atom, and it has a positive electrical charge of one and a mass of 1 atomic mass unit. Together with neutrons, protons make up virtually all of the mass of an atom. Neutrons, on the other hand, have no charge and have a mass that is slightly greater than a proton.
The Dalton (abbreviated Da) is a unit used in expressing atomic or molecular mass, defined as 1/12 the mass of a single atom of carbon-12 (i.e., a carbon atom containing 6 protons and 6 neutrons). As a consequence of this definition and the fact that electrons have very small mass relative to protons and neutrons, the dalton is approximately equal to the mass of one nuclear particle: a proton weighs 1.009 Da, whereas a neutron weighs 1.007 Da.
One dalton is a unit of mass that is almost unfathomably small; 1 gram is equivalent to 6.02 x 1023 daltons. Accordingly, the Dalton is only suitable for measuring the mass of objects on the molecular scale.
Proton Mass to Dalton Conversion Table
The following table provides values to convert Proton mass to dalton, or vice versa:
| Proton Mass | Dalton |
|---|---|
| 0.01 Proton mass | 0.0100728267 dalton |
| 0.1 Proton mass | 0.1007282675 dalton |
| 1 Proton mass | 1.0072826748 dalton |
| 2 Proton mass | 2.0145653496 dalton |
| 3 Proton mass | 3.0218480244 dalton |
| 5 Proton mass | 5.036413374 dalton |
| 10 Proton mass | 10.0728267481 dalton |
| 20 Proton mass | 20.1456534962 dalton |
| 50 Proton mass | 50.3641337404 dalton |
| 100 Proton mass | 100.7282674808 dalton |
| 1000 Proton mass | 1007.2826748078 dalton |
How to Convert Proton Mass to Dalton
To convert Proton mass to Dalton, use the following formula:
1 Proton mass = 1.0072826748 dalton
1 dalton = 0.9927699791 Proton mass
Example:
To convert 15 Proton mass to dalton, use the formula:
15 Proton mass = 15 x 1.0072826748 dalton
Therefore, 15 Proton mass = 15.1092401221 dalton.
Popular Weight and Mass Unit Conversions
It is also possible to convert Proton mass to other units of weight and mass. Popular weight and mass units include grams, kilograms, pounds, ounces, milligrams, and micrograms.
Related Terms
When talking about the mass of a proton, other related terms include Mole, Mass Spectrometry, Mass-to-Charge Ratio, m/z, Isotope, 1H, 2H, Deuterium, 3H, Tritium, 12C, Carbon-12, 13C, Carbon-13, 14C, Carbon-14, Nominal Mass, and Exact Mass.
To sum up, the mass of a proton is approximately 1 dalton. This is because a dalton is defined as 1/12 the mass of a single atom of carbon-12, and a proton has approximately the same mass as a carbon-12 atom. It is also possible to convert Proton mass to other units of weight and mass, such as grams, kilograms, pounds, ounces, milligrams, and micrograms. Other related terms include Mole, Mass Spectrometry, Mass-to-Charge Ratio, m/z, Isotope, 1H, 2H, Deuterium, 3H, Tritium, 12C, Carbon-12, 13C, Carbon-13, 14C, Carbon-14, Nominal Mass, and Exact Mass.




Leave a Comment