Understanding the Unit of Atomic Mass

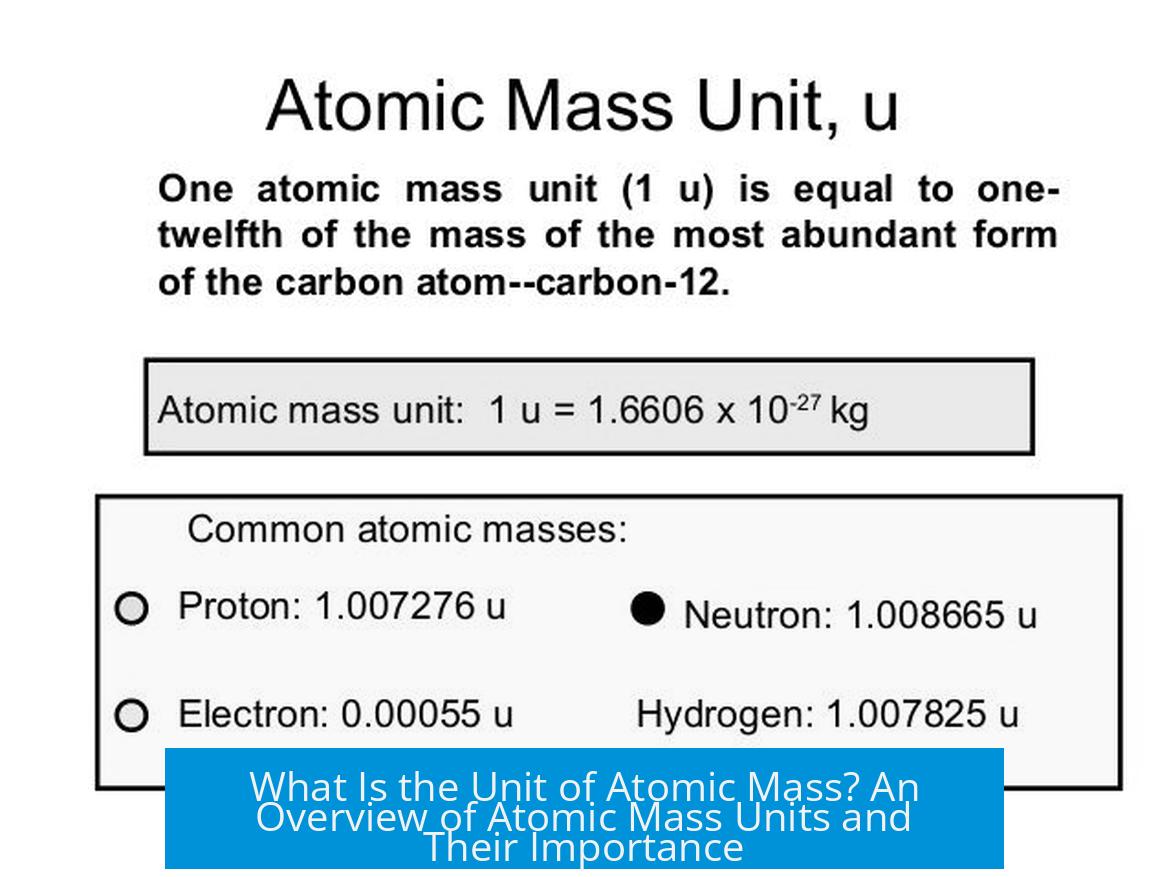
The unit of atomic mass is known as the atomic mass unit (a.m.u.), also called the Dalton. These two terms are used interchangeably to represent the mass of atoms and subatomic particles. The atomic mass unit provides a scale for expressing masses of particles that are too small to measure conveniently in kilograms or grams.
What Is an Atomic Mass Unit (a.m.u.)?
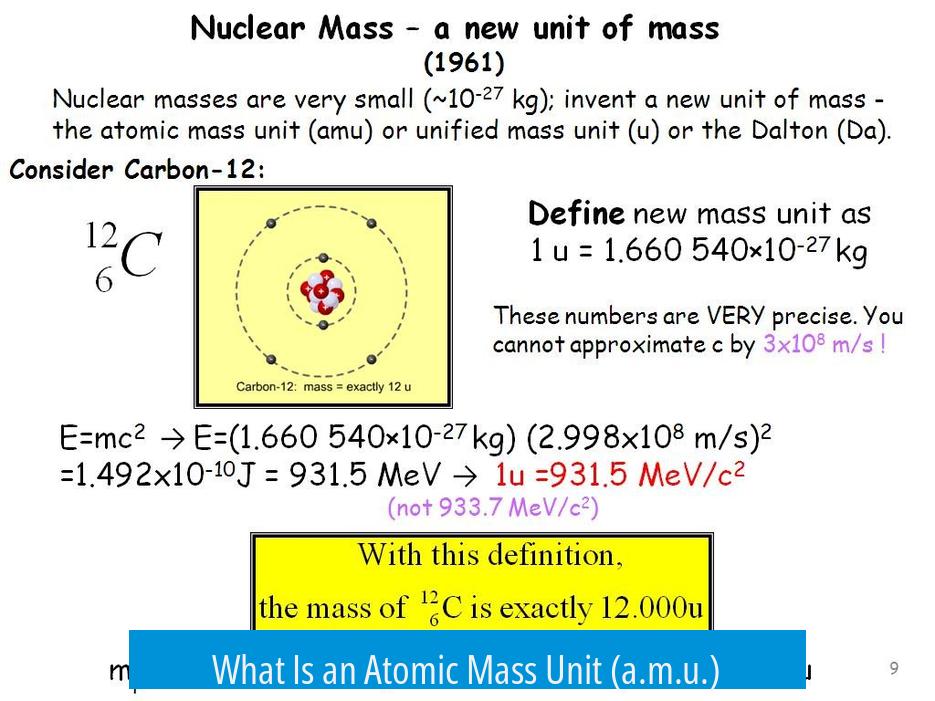
The atomic mass unit quantifies the mass of atoms, molecules, and subatomic particles such as protons and neutrons. It serves as a scaled unit that allows scientists to discuss atomic masses without resorting to extremely small decimal numbers in kilograms or grams.
Historically, the atomic mass unit related directly to a fraction of the mass of a specific isotope of carbon, carbon-12. The unit was defined as one-twelfth of the mass of one atom of carbon-12. This provided a consistent baseline because carbon-12 is a stable isotope that occurs naturally in fixed proportions.
Dalton: Another Name for Atomic Mass Unit
- The Dalton (symbol Da) is a unit exactly synonymous with the atomic mass unit.
- Biochemists and molecular biologists often use Daltons when describing molecular masses, especially of proteins and nucleic acids.
- Both a.m.u. and Dalton describe the same quantity: the average mass of a nucleon (neutron or proton) inside an atom.
Recent Changes in the Definition of Atomic Mass Unit
The atomic mass unit definition has been updated recently to reflect advances in measurement precision and standards in physics. Previously, the unit was tied explicitly to the mass of carbon-12 atoms. Now, it is defined as a fixed numerical value independent of any physical reference.
More specifically:
- The current definition assigns a fixed value to the atomic mass unit rather than referring directly to the carbon isotope.
- This update aligns the atomic mass unit with the redefinition of other SI units to fixed numerical constants, improving reproducibility and precision.
- The change occurred within the last year, reflecting ongoing refinement in fundamental constants and measurement standards.
Practical Measurement: How Atomic Mass Unit is Used
Measuring an atom’s mass directly is impossible with traditional scales because atoms are extremely small.
Masses of atoms are practically deduced by considering the mass of one mole of atoms instead of single atoms or molecules.
- One mole corresponds to Avogadro’s number (6.022×1023) of entities.
- The mass of one mole of carbon-12 atoms is measured and used to infer the atomic mass unit scale.
- Essentially, the atomic mass unit is a conversion factor to connect macroscopic masses (measured in kilograms or grams) to microscopic atomic masses.
For example, dividing the macroscopic mass of one mole of atoms by Avogadro’s number yields the mass of a single atom in kilograms. Dividing that value by the conversion factor (which expresses kilograms per atomic mass unit) gives the mass in atomic mass units.
Formal Definition and Unit Context
Atomic mass units function not as direct measurements but as conversion factors, much like other physical units.
Understanding the atomic mass unit requires writing it out with proper units:
1 a.m.u. = (mass of one mole of carbon-12 atoms in kg) / (6.022×1023 atoms × 1 a.m.u. factor)
This expression emphasizes that the atomic mass unit links macroscopic masses (kilograms or grams) to the microscopic scale using Avogadro’s number.
- This approach is analogous to other unit definitions:
- A mole represents 6.022×1023 molecules per mole, a conversion factor between quantity and amount.
- A volt represents 1 joule per coulomb, a conversion factor for energy per charge.
- A meter is defined as the distance traveled by light in vacuum in a specific fraction of a second.
Relation to Other Units and Importance in Science
The atomic mass unit enables scientists to communicate about atomic and molecular masses on a convenient scale that facilitates calculations in chemistry, physics, and biology.
| Unit | Symbol | Use | Relation |
|---|---|---|---|
| Atomic Mass Unit (Dalton) | a.m.u. or Da | Mass of atoms, molecules, nucleons | Approximately 1.6605 × 10−27 kg |
| Kilogram | kg | SI base unit of mass | 1 kg = ~6.022×1023 × 1 a.m.u. |
| Mole | mol | Amount of substance (count) | 6.022×1023 entities/mol |
The atomic mass unit allows conversion between microscopic particle masses and macroscopic scales used in laboratories. This interaction is essential for calculating molecular weights, reaction yields, and stoichiometric relationships in chemistry.
Summary of Key Points
- The unit of atomic mass is the atomic mass unit (a.m.u.), also called the Dalton.
- The a.m.u. historically tied to carbon-12’s mass but recently redefined as a fixed constant.
- The unit serves as a conversion factor linking atomic or molecular masses (in kilograms or grams) to a convenient scale for atoms and particles.
- Practically, atomic masses are deduced from the mass of 1 mole of carbon-12 atoms, using Avogadro’s number as a key figure.
- The atomic mass unit is critical in fields like chemistry and physics for measuring and comparing the masses of very small particles consistently.
- Comparisons with other units highlight its fundamental role as a conversion factor in the system of measurements.
What is the atomic mass unit (a.m.u.)?
The atomic mass unit is a unit of mass used for atoms and subatomic particles. It is also called a Dalton. Both terms mean the same thing and are used interchangeably.
Has the definition of the atomic mass unit changed recently?
Yes. The atomic mass unit is no longer defined based on the mass of carbon-12. It is now a fixed number, independent of any specific element.
How is the atomic mass unit practically measured?
It is derived from the mass of one mole of carbon-12 atoms, not a single atom. This is because measuring the mass of a single atom directly is difficult.
Why is the atomic mass unit considered a conversion factor?
The atomic mass unit converts mass in kilograms or grams to a unit suited for atoms. Dividing the mass of an atom by this factor gives its mass in a.m.u.
How does the atomic mass unit relate to other unit definitions?
Like a mole or a volt, the atomic mass unit is best understood as a conversion factor with units. It provides a link between atomic scale masses and macroscopic mass units.




Leave a Comment