Fun Facts about Strontium Carbonate

Strontium carbonate is notable for producing a bright fuchsia flame when burned in methanol. This distinctive color makes it useful for color effects in pyrotechnics and flame tests.
Flame Color
- Burning strontium carbonate in methanol yields a vivid fuchsia flame.
Industrial Use: Vacuum Tube Cathodes
Strontium carbonate combines with barium carbonate to produce an oxide coating for vacuum tube cathodes. This coating improves electron emission in old vacuum tubes, enhancing their performance in electronics.
Biological Roles of Strontium

Though not essential in large quantities, strontium plays interesting roles in marine biology. Corals incorporate strontium partly into their calcium-based skeletons. It also participates in their immune systems and is found in some algae species.
- Used partially by corals for skeletal structure.
- Involved in coral immune defenses.
- Present in certain algae.
Aquatic Tank Considerations
Strontium carbonate’s solubility is low. When dosing aquatic tanks, incorrect amounts can cause it to precipitate. This limits its use in maintaining strontium levels in aquarium water.
Homeopathic Reference (Satirical)
Take strontium carbonate, prepare a solution, dilute repeatedly, and after mystical rituals claim a cure for arthrosis and sclerosis. This humorous take mocks homeopathic exaggerations.
General Characterization

Strontium carbonate is a simple, basic compound without complex chemistry. Despite that, the unique flame color and biological roles add interesting facets to its profile.
Key Takeaways
- Burns with bright fuchsia flame in methanol.
- Used with barium carbonate in vacuum tube cathodes.
- Incorporated by corals and some algae biologically.
- Low solubility can cause precipitation issues in tanks.
- Subject of humorous homeopathic references.
Anyone Know Any Fun Facts About Strontium Carbonate?
Strontium carbonate may sound like a dull chemical formula, but it’s actually a little fireworks show waiting to happen! Ever wondered what happens when you burn this compound in methanol? You get a bright fuchsia flame. That’s not your everyday fire color.
So, what’s the deal with strontium carbonate beyond its flashy fire trick? Let’s dive into some truly fascinating—and sometimes quirky—facts about this compound that might just make you the hit at your next science trivia night.
A Flash of Color: The Flame Test

Throw strontium carbonate into a methanol flame, and watch as it burns bright fuchsia. That intense pinkish hue? It happens because strontium ions emit light at specific wavelengths when heated. It’s the chemical equivalent of a diva stepping into the spotlight.
Fireworks makers love strontium carbonate for this very reason. If you have ever admired dazzling red or pink fireworks on Independence Day, strontium’s vibrant fire glow is probably what you were watching. So next time you see those festive colors, you can thank strontium carbonate!
Old-School Tech: Vacuum Tube Cathodes
Strontium carbonate’s fame isn’t limited to celebrations. Remember vacuum tubes? Those bulky, glowing glass bulbs that powered radios and early TVs? Well, strontium carbonate was part of the secret sauce in those.
Alongside barium carbonate, it formed an oxide coating on vacuum tube cathodes. This coating helped electrons flow properly, making devices work smoothly. While vacuum tubes may seem ancient today, their role in jumpstarting electronics technology is crucial—and strontium carbonate was right there behind the scenes.
Strontium in Nature: More Than Just a Chemical
Strontium carbonate is a chemical compound, but the element strontium itself has some charming natural gigs. Coral reefs? Yep, strontium helps build their skeletons. Corals cleverly incorporate strontium into their calcium carbonate structures, strengthening those vibrant underwater cities.
Plus, it plays a subtle role in immune systems—not just in one species, but across some algae and marine life. Think of it as a tiny sidekick helping sea creatures’ defenses work better. Scientists are still untangling its full biological roles, but it’s clear strontium isn’t just passive; it’s a quiet contributor.
Careful With Those Doses in Aquariums
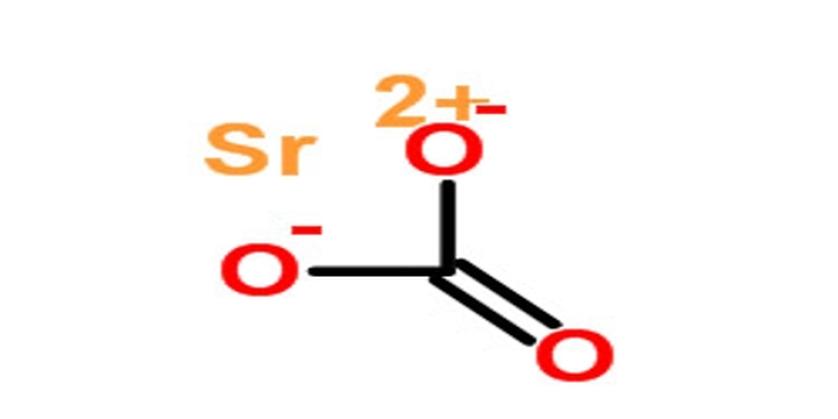
Got a reef aquarium? Strontium carbonate can be your friend or foe. It’s sometimes added as a supplement to create a healthy environment for corals and other aquatic creatures.
However, be warned: if you mess up the dosage, strontium carbonate can fall out of solution and turn into a cloudy crust of unwanted solids. Picture your carefully balanced tank suddenly getting a bit messy—nobody wants that! Precision is key.
The Homeopathic Side—With a Wink
Here’s a cheeky one: some homeopathic remedies tout “strontium carbonicum” as a cure-all for arthrosis and sclerosis.
The process? Dilute the salt so many times it’s basically a whisper of a whisper. Add a ritual like singing to it, lighting incense, and voila—profit!
While this might raise a smile or an eyebrow, it’s a great example of how scientific compounds sometimes get wrapped in mystical stories far beyond their real chemistry.
Is Strontium Carbonate Boring?
It’s easy to say strontium carbonate is “kinda basic,” literally and figuratively. Chemically, it’s a simple salt—basic in pH, and stable as a rock.
But its unassuming nature hides a fascinating story across fields from fireworks to marine biology.
Let’s sum it up:
- Bright fuchsia flames: a chemist’s favorite party trick.
- Old tech hero: key in vacuum tube cathodes.
- Nature’s helper: forms coral skeletons and supports immune systems in marine life.
- Aquarium boss: tricky yet beneficial for reef tanks.
- Quirky homeopathic lore: a wink to alternative medicine.
So, strontium carbonate might look basic, but it’s packed with fun facts and practical uses that keep it surprisingly relevant in science and industry.
Have you ever seen strontium carbonate in action?
Maybe you’ve caught the fuchsia glow at a fireworks show or kept an aquarium with a hidden dosing secret. If not, perhaps now’s your time to start appreciating the humble compound that’s quietly dazzling without much fuss.
Strontium carbonate is a reminder that even the simplest chemicals have stories worth telling. Next time someone calls it boring, you can share these spotlight moments and change their minds.
Want to test its flame color yourself, or curious about how it fits into modern tech and ecology? Dive in—strontium carbonate is more than meets the eye!
What color does strontium carbonate produce when burned?
Burning strontium carbonate in methanol creates a bright fuchsia flame. This makes it useful for producing red colors in fireworks and flares.
How is strontium carbonate used in vacuum tubes?
It is combined with barium carbonate to create oxide coatings on cathodes in vacuum tubes. This improves electron emission and tube performance.
Does strontium carbonate have any role in marine life?
Strontium helps corals build their skeletons and also plays a part in the immune systems of corals and some algae.
What happens if strontium carbonate dosage is incorrect in aquatic tanks?
It can fall out of solution, causing issues in tank water chemistry. Proper dosage is needed to maintain stability.
Is strontium carbonate used in homeopathy?
Satirically, it is diluted repeatedly and sold as “Strontium carbonicum” with unfounded healing claims, highlighting skepticism about homeopathic practices.





Leave a Comment