Which Type of Bond Is Strongest: Ionic or Covalent?

In general, ionic bonds are stronger than covalent bonds when considering the energy required to break them under ideal conditions. This conclusion arises from comparing the physical quantities used to measure the strength of these bonds: lattice energy for ionic bonds and bond dissociation energy for covalent bonds.
Understanding Bond Strength
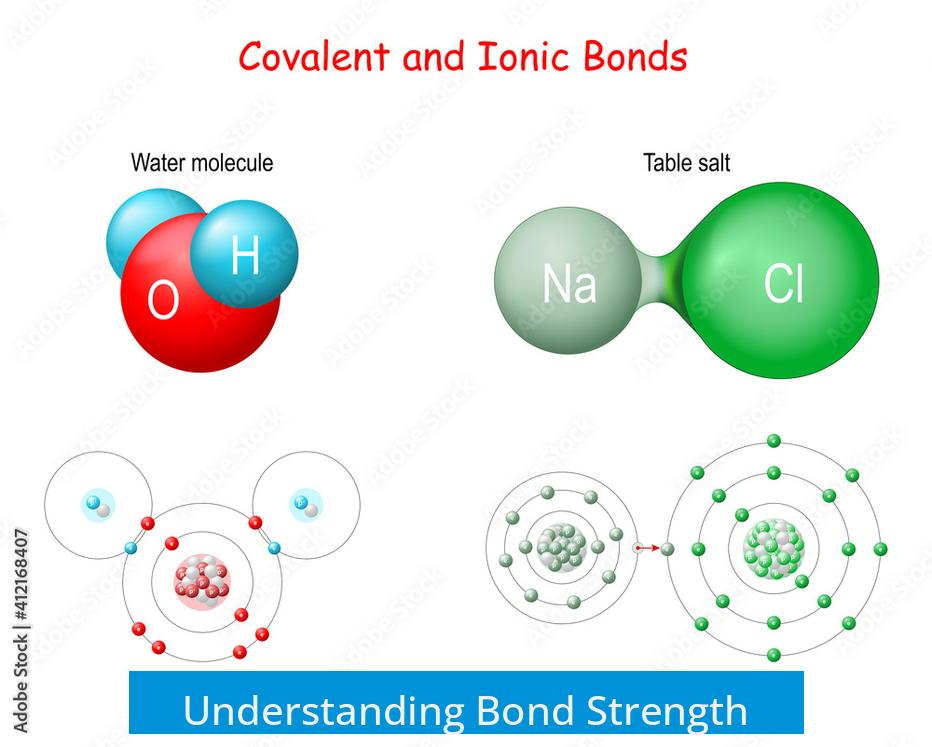
Bond strength is a measure of how much energy is needed to break a bond. For covalent bonds, this is quantified by bond dissociation energy. This refers to the energy required to homolytically cleave the bond into two radicals, separating them infinitely in a vacuum.
For ionic bonds, strength is described by lattice energy. This is the energy necessary to separate an ionic crystal into its individual ions, moving them to infinite distance, also in vacuum conditions.
Comparing Energy Values
| Bond Type | Energy Range (kJ/mol) | Example |
|---|---|---|
| Covalent Bonds | 150 to 1080 | Iodine (I–I) ~150; Carbon monoxide triple bond ~1080 |
| Ionic Bonds (Lattice Energy) | 770 to 2900+ | NaCl ~770; MgO ~2900 |
For example, the I–I bond has a bond dissociation energy of about 150 kJ/mol, while the CO triple bond reaches 1080 kJ/mol. By contrast, the lattice energy of common ionic compounds ranges from 770 kJ/mol for NaCl to as high as 2900 kJ/mol for MgO. This reveals ionic bonds, in their crystalline lattice form, are often stronger than typical covalent bonds on this scale.
Context Matters: Vacuum vs. Solution
These measurements occur in a vacuum, an environment far removed from most chemical contexts. In practical chemistry, especially medicinal chemistry, conditions involve solutions. Here, solvation plays a crucial role.
Solvation energy reduces the apparent strength of ionic bonds by stabilizing separated ions. This allows ionic compounds to dissociate readily in solution.
Behavior of Bonds in Solution
- Ionic bonds break apart easily due to interactions with solvent molecules.
- Covalent bonds largely remain intact, maintaining molecular integrity.
- Medicinal chemists often consider ionic bonds weaker because they dissociate quickly in biological fluids.
For example, a primary amine salt like NH3+Cl- dissociates in solution, releasing free amines. This dissociation reduces the effective presence of ionic bonding in biological systems.
Relevance in Biological and Medicinal Chemistry
Despite ionic bond strength in vacuum, covalent bonds dominate biological mechanisms. The action of drugs depends almost exclusively on covalent bonds, which hold together the drug’s core structure.
Ionic bonds often assist solubility or transport rather than maintain structural integrity. Hence, covalent bonds commonly determine biological activity and chemical behavior in living organisms.
Extended Lattices: Diamond vs. Salt
An interesting comparison lies in extended lattices. Diamond’s covalent lattice is extremely strong and rigid, giving it remarkable hardness and durability. NaCl, with an ionic lattice, also exhibits high lattice energies but differs in physical properties like brittleness and solubility.
This comparison shows covalent lattices can also be extraordinarily strong, but the nature and implications of strength vary based on the chemical environment and structure type.
Defining “Strongest” Bond
The concept of bond strength depends on definition. If strength refers to energy needed to break bonds in vacuum, ionic bonds generally surpass covalent bonds. However, in solution or biological conditions, solvation and molecular context change how bonds behave.
Thus, “strongest” depends on context: physical chemistry favors ionic bond strength via lattice energy; biological systems emphasize covalent bond stability.
Key Takeaways
- Ionic bonds have higher lattice energies in vacuum than typical covalent bond dissociation energies.
- Covalent bond strength varies widely, with some bonds (e.g., triple bonds) nearly matching or exceeding weaker ionic bonds.
- Solvation effects in solution reduce the apparent strength of ionic bonds significantly.
- Covalent bonds largely remain intact in biological systems and are critical to drug function.
- The definition of “strongest” bond must consider experimental conditions and context.
So, at the End, Which Type of Bond Is the Strongest, Ionic or Covalent?
Let’s cut to the chase: ionic bonds are generally stronger than covalent bonds when measured in a vacuum. That’s the straightforward chemistry headline. But, as with many things in science and life, the story doesn’t end there. It all depends on how you define “strongest,” the environment you’re talking about, and what practical scenario you’re investigating.
What Makes a Bond “Strong” Anyway?
Strength in bonds is not just a buzzword thrown around at chemistry parties. It has a precise meaning. For covalent bonds, strength is measured by bond dissociation energy. This is the energy you’d need to break that bond into two separate radicals and whisk them off to infinity without any attraction.
For ionic bonds, the equivalent measure is lattice energy. This refers to the energy required to separate a crystal made of ions into individual ions and move them infinitely far apart, like a social distancing mandate on ions.
To put numbers on the table, covalent bond dissociation energies can range from about 150 kJ/mol for a weak iodine-iodine bond to a whopping 1080 kJ/mol for a carbon-oxygen triple bond. Meanwhile, ionic bonds can boast lattice energies starting around 770 kJ/mol for everyday salt (NaCl) and escalating dramatically up to 2900 kJ/mol for compounds like magnesium oxide (MgO).
Covalent vs Ionic: Numbers Tell a Clearer Tale—Sometimes
| Bond Type | Strength Measure | Energy Range (kJ/mol) | Example |
|---|---|---|---|
| Covalent Bond | Bond Dissociation Energy | 150 – 1080 | Iodine-Iodine (I—I), Carbon-Oxygen Triple Bond (CO) |
| Ionic Bond | Lattice Energy | 770 – 2900 | NaCl, Magnesium Oxide (MgO) |
From those figures alone, ionic bonds, especially as extended lattices, pack a stronger punch than typical single covalent bonds in isolation.
But Wait! What Happens When You Step Out of The Vacuum and Into Reality?
You might be thinking: “Chemistry in a vacuum? That’s like judging a bike race on paper instead of the track.” Exactly. In the real world, especially in solutions like water—which is pretty much every biological environment—things get trickier.
Here’s why: ionic bonds come with a catch called solvation energy. Water molecules or other solvents gather around ions and essentially soften the ionic bond’s grip, making them more amenable to breaking apart. This is why salt dissolves so easily in water despite its strong lattice energy.
In medicinal chemistry, this fact is a game-changer. An ionic bond in a salt, say, between a positively charged amine and a chloride ion, is fleeting. As soon as it hits a watery solution, it breaks apart. The free amine goes about its business, functioning as intended. On the flip side, the covalent bonds inside that drug molecule rarely break apart in solution; they hold the structure together.
This leads to something that sounds paradoxical but is true in a practical sense: many medicinal chemists consider covalent bonds to be stronger in biological contexts because they actually matter and persist, while ionic bonds dissolve away.
Diamond versus Salt: A Tale of Two Lattices
To grasp the difference in strength dynamics, look at two famous “extended lattice” materials: diamond and table salt. Diamond is a giant covalent lattice with each carbon atom bonded covalently to four others. Table salt, on the other hand, is an ionic lattice composed of Na+ and Cl− ions held by ionic bonds.
Diamond’s extreme hardness and chemical stability showcase the power of extended covalent lattices. Meanwhile, salt crystals, although strong in a dry state, crumble easily in water because their ionic bonds break down in solution.
The Crux: Defining “Strongest”
So, what is the strongest bond? It depends on your definition.
- If you measure bond strength in a vacuum, without interference or external forces, the ionic bond—thanks to high lattice energy—wins.
- If you consider typical real-world environments like water or biological fluids, covalent bonds usually prevail because they resist breaking down under those conditions.
- In extended networks, covalent lattices like diamond create colossal durability at a larger scale, while ionic lattices offer different strengths and weaknesses, including solubility and brittleness.
Think of it like this: ionic bonds are like strong magnets sticking together in a dry room—hard to pull apart. But throw them into a pool full of water molecules and their grip loosens. Covalent bonds, meanwhile, are like glued joints that hold fast regardless of moisture.
Why Should You Care?
This isn’t just academic hair-splitting. Understanding bond strength helps in:
- Drug design: knowing which bonds will hold in your body is crucial.
- Material science: designing materials for hardness, flexibility, or solubility.
- Everyday chemistry: like why salt dissolves but diamond doesn’t.
So next time someone throws you the question, “Which bond is stronger, ionic or covalent?” you can answer boldly and contextually:
“Ionic bonds are stronger in theory and vacuum, but in practical, real-world contexts—especially in solution and biology—covalent bonds often trump ionic bonds because they endure the chaotic environment.”
Parting Tips for the Curious Chemistry Buff:
- Explore lattice energy and bond dissociation energy yourself. Both are measurable and fascinating!
- Remember the environment is king; bonds behave very differently in water versus air.
- If you’re in medicinal chemistry or biology, think covalent for stability, ionic for temporary interactions.
- For fun, compare materials like salt and diamond to see how bonding type changes properties.
Ultimately, chemistry’s strongest bonds depend on the rules of engagement: the battlefield is either the vacuum or the busy solution. And that’s science with a twist.
Ready to rethink bonds and impress your friends with some chemistry wisdom at the next gathering? Remember: context is everything.
1. Which bond is stronger in a vacuum, ionic or covalent?
Ionic bonds are generally stronger in a vacuum. Their lattice energies can be much higher than covalent bond dissociation energies.
For example, MgO has a lattice energy of about 2900 kJ/mol while typical covalent bonds range from 150 to 1080 kJ/mol.
2. How do you measure the strength of a covalent bond?
Covalent bond strength is measured by bond dissociation energy. This is the energy needed to split one bond homolytically into radicals and separate them completely.
Bond dissociation energies vary widely depending on the bond type.
3. Why might ionic bonds seem weaker in biological systems?
In solutions like the body, ionic bonds often break due to solvation. Water molecules surround and stabilize ions, causing the bond to dissociate easily.
Thus, ionic bonds appear weaker in this context, while covalent bonds remain intact.
4. Do covalent bonds play a bigger role in drug action than ionic bonds?
Yes. Most drug actions rely on covalent bonds since they persist in physiological environments.
Ionic bonds often just help with solubility and do not impact the drug’s key interactions.
5. Can comparing diamond and salt help understand bond strength?
Diamond has an extended covalent lattice, making it very hard. Table salt has an ionic lattice that can dissolve easily in water.
This shows covalent lattices can be very strong, but context matters for real-world strength.



Leave a Comment