What Is H3O+ from Positive Oxygen?
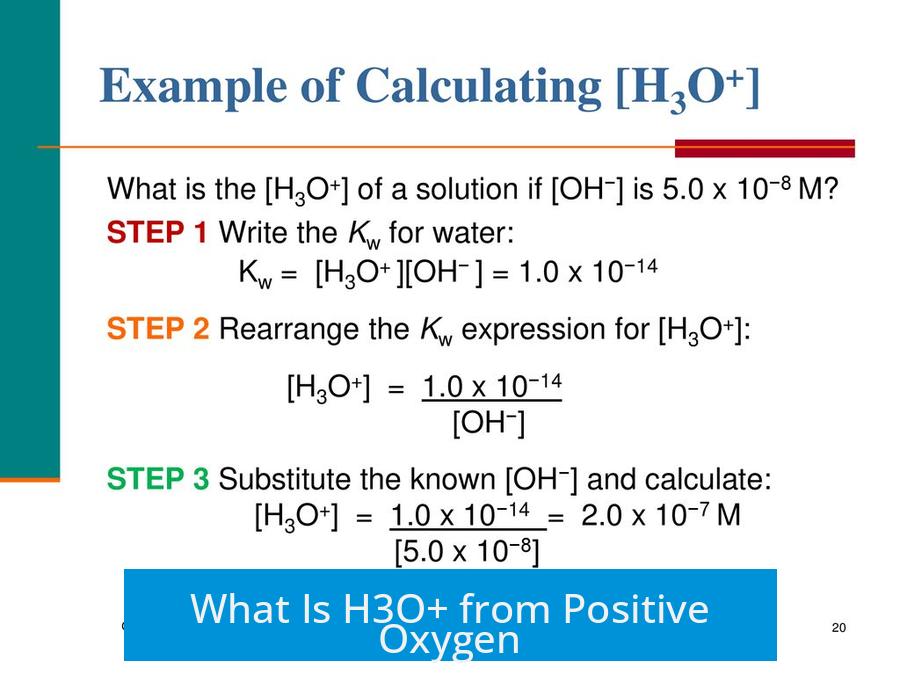
The hydronium ion (H3O+) is a positively charged species formed when a proton (H+) attaches to a water molecule (H2O), resulting in a molecule with an overall positive charge distributed over the entire ion rather than localized on oxygen alone.
Nature and Formation of the Hydronium Ion
In aqueous acidic solutions, free protons exist only transiently and rapidly associate with water molecules. This association yields hydronium ions, denoted H3O+. The ion represents the protonated form of water, central to acid-base chemistry in water.
Besides the simple H3O+ ion, more complex hydrated forms can appear, such as H9O4+, indicating dynamic cluster structures in solution. However, the protonation of a single water molecule remains the fundamental event producing H3O+.
Charge Distribution of H3O+
The notation (H3O)+ clarifies that the positive charge is not localized on the oxygen atom alone. Instead, the charge is spread over the entire ion. Oxygen does not carry a full positive charge separate from hydrogen atoms.
This is because the positive charge results from the addition of a proton devoid of electrons. The whole entity shares the charge, with electron density adjusting to it.
Protonation Mechanism: Oxygen’s Role
Acids donate protons (H+ ions) to bases. Water molecules act as bases by accepting these protons. The question is how this proton binds to water.
Oxygen in water has two lone pairs (non-bonding electron pairs). One lone pair forms a coordinate covalent bond with the proton. Since the proton has no electrons, both electrons in this new bond come from oxygen.
This bond formation creates H3O+. The water molecule “shares” one of its electron pairs with the proton, stabilizing it.
Why Does Oxygen Appear Positively Charged?
Even though the overall hydronium ion has a positive charge, oxygen may appear to hold partial positive character. Normally, oxygen has six valence electrons. Upon bond formation with H+, it donates an electron pair completely.
This donation leaves oxygen effectively electron-deficient by approximately one electron compared to neutral oxygen. Thus, oxygen may be viewed as carrying a partial positive charge, though strictly, the positive charge is on the whole ion.
Equilibrium in Aqueous Solutions
Water molecules exist in equilibrium with protons and hydronium ions:
H2O + H+ ⇌ H3O+
This equilibrium governs acid-base behavior in aqueous media. Free protons rarely exist alone but rather “assigned” to water molecules as H3O+ or even larger hydrated forms.
Summary Table: H3O+ from Positive Oxygen
| Aspect | Description |
|---|---|
| Formation | Proton (H+) binds to water molecule through oxygen’s lone electron pair, producing hydronium ion. |
| Charge Distribution | Positive charge is delocalized over entire H3O+, not solely on oxygen atom. |
| Oxygen’s Role | Shares its lone pair electrons with proton forming a coordinate covalent bond. |
| Partial Positive Character | Oxygen donates electrons; appears electron-deficient, hence partially positive within the ion. |
| Equilibrium | Water and protons exist in balance with hydronium, defining acidity in aqueous solution. |
Key Takeaways
- H3O+ forms by protonation of water via oxygen’s lone electron pairs.
- The positive charge is shared over the entire hydronium ion, not localized on oxygen.
- Oxygen donates both electrons in the bond to the proton, appearing partially electron-deficient.
- Hydronium is fundamental to acid-base chemistry in aqueous solutions.
- Water maintains equilibrium with protons and hydronium ions, explaining the nature of acidity.
What exactly is the H3O+ ion in relation to positive oxygen?
H3O+ is the hydronium ion formed when water accepts a proton (H+). The positive charge is on the whole molecule, not just on the oxygen atom.
How does oxygen in water contribute to forming H3O+?
The oxygen atom has two lone pairs of electrons. One lone pair bonds with the proton, creating the hydronium ion through a coordinate covalent bond.
Why does oxygen appear to carry a positive charge in H3O+?
Oxygen donates an electron pair to bond with H+, which has no electrons to share. This effectively reduces oxygen’s electron count, making it seem positively charged.
Is the positive charge localized on oxygen or spread over H3O+?
The positive charge is distributed over the entire H3O+ ion. It is incorrect to think of the oxygen alone as carrying the charge.
How is H3O+ formed from acid-base reactions involving water?
Acids donate protons (H+) to water molecules. Water accepts these protons using oxygen’s lone pairs, producing H3O+ ions in solution.
Can the structure of H3O+ be more complex than just one protonated water?
Yes, in solution more complex species like H9O4+ exist, which involve multiple water molecules and protons forming hydrated ions.




Leave a Comment