Potency of Dyes: Understanding Methylene Blue Concentration in Water
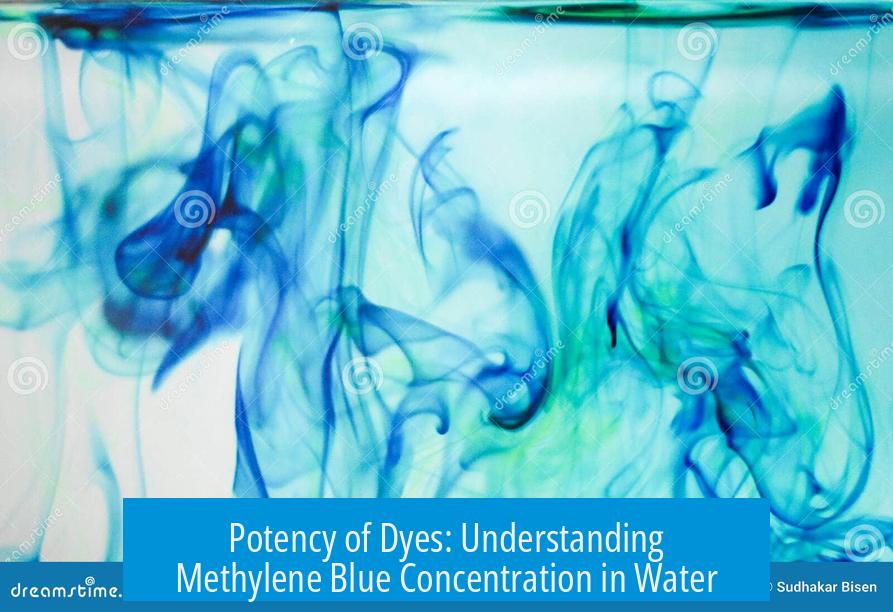
Methylene blue is an extremely potent dye, demonstrated by the fact that 10 to 30 grams dissolved in 20,000 liters of water can produce a visible and impactful coloration. This concentration translates roughly to about 1 milligram per liter (mg/L) on average, showcasing the remarkable potency of methylene blue even in highly dilute solutions.
General Dye Potency
Dyes like FD&C blue #20 and red #40 come in powdered form and are known for their intense coloring power. For instance, solid powders of these dyes require only tiny quantities to achieve significant coloration, making their handling relevant in both industrial and laboratory settings.
- Methylene blue’s staining power is notable. An accidental spill of 80 liters of concentrated dye at a workplace often results in visible blue stains over large areas.
- Even small amounts are sufficient to color large water bodies. Anecdotes report that just a few grams could theoretically dye entire lakes, underscoring the dye’s potency.
Specifics of Methylene Blue Potency
Considering the example of 10-30 grams of methylene blue in 20,000 liters, the range may appear broad, but in practical terms, the effect does not vary dramatically within this scope. A median of 20 grams equates to approximately 1 mg/L or 4.6 micromolar (μM).
| Amount of Methylene Blue (g) | Volume of Water (L) | Concentration (mg/L) | Approx. Molarity (μM) |
|---|---|---|---|
| 10 | 20,000 | 0.5 | 2.3 |
| 20 | 20,000 | 1.0 | 4.6 |
| 30 | 20,000 | 1.5 | 6.9 |
The extinction coefficient of methylene blue, around 95,000 L·mol−1·cm−1, indicates it absorbs light strongly, supporting its ability to color large volumes of water with little substance. Some dyes have higher coefficients (e.g., 400,000), but methylene blue ranks as a notably vivid dye.
Effects and Handling of Methylene Blue

Skin and Staining
Methylene blue stains both intact and damaged skin. Accidental exposure can leave vivid blue or greenish-blue markings:
- Needle stick accidents during preparation can create temporary blue “tattoos” on fingers that last weeks.
- Staining especially affects dead or damaged skin such as cuts or eczemas.
- Cleaning stains involves reducing methylene blue to its leuco form using ascorbic acid or sodium ascorbate. This form is colorless and water soluble but re-oxidizes upon air exposure, so thorough washing is necessary.
Spills may leave persistent stains; for example, 10 ml of methylene blue antidote spilled in a storage area stained the floor blue for six years, illustrating its durability.
Medical and Toxicological Aspects
Methylene blue has significant medical uses, such as treating methemoglobinemia. Intravenous doses range from 1-2 mg per kg of body weight, topping at about 50 mg per administration.
Its potency relates not only to color but to biology. Methylene blue acts as a monoamine oxidase inhibitor (MAOI) at certain concentrations.
One reported case describes methylene blue used during open-heart surgery causing blue discoloration on the patient’s skin by diffusion through tissues, highlighting its potent bioactivity even in small quantities.
Environmental and Practical Considerations

Use in Water Systems
Adding 10-30 grams of methylene blue to waterparks or pools (20,000 liters) yields visible blue tinting, sufficient to alter water appearance without introducing visible pollution markers. At these concentrations, the dye is not classified as a significant pollutant.
However, some organic compounds containing benzene rings become pollutants at concentrations less than 1 microgram per liter (μg/L or 0.001 mg/L). By comparison, this methylene blue concentration (1 mg/L) is 1000 times higher than allowable limits for such pollutants in potable water, warranting careful consideration.
Environmental Impact and Remediation
Experts in environmental remediation sometimes find dye contamination troubling due to its persistence and staining ability. For instance, singlet oxygen generated through photochemical processes can degrade pool liners by making small holes when methylene blue is present.
Thus, keeping dye levels low is critical to preserving infrastructure and aquatic environmental quality.
Summary of Key Points

- Methylene blue is extremely potent; 10-30 g in 20,000 L of water produces visible coloring at ~1 mg/L.
- Its extinction coefficient (~95,000) reflects strong light absorption and dye capability.
- Methylene blue stains skin and surfaces profoundly; removal requires reducing agents and thorough cleaning.
- Medical doses for treatment of methemoglobinemia are in mg/kg range, underpinning its bioactivity.
- Environmental concentrations well above μg/L require careful management to avoid pollution or damage.
- Potential exists for infrastructure degradation, such as pool liners, from photochemical reactions involving methylene blue.
Some Dyes Are Extremely Potent: The Curious Case of 10–30g of Methylene Blue in 20,000L of Water
Yes, some dyes are so insanely potent that a mere 10 to 30 grams of methylene blue can color a whopping 20,000 liters of water. If that doesn’t sound mind-boggling, hold tight—your bathtub, swimming pool, or even a lake could take on a brilliant blue hue from what seems like a minuscule amount of powder. But why is methylene blue so powerful? And what should you know about using or handling such compounds? Let’s dive in—blue goggles on.
The Astonishing Potency of Methylene Blue
First off, methylene blue isn’t your everyday kitchen dye. We’re talking about a solid powder so potent that just a few grams go viral with color. Imagine having 2 kilograms of FD&C blue #20 or 1 kilogram of red #40 powder. That’s an absurd level of strength in solid form. In fact, methylene blue is the dye equivalent of a whisper that echoes across an auditorium.
Consider this: spilling 80 liters of concentrated methylene blue isn’t just a clumsy accident—it’s a recipe for a ten-year-old chemistry set nightmare. Just a few flakes spilled on a bathroom counter can make that spot blue for years. A laboratory veteran once joked, “My legacy there was literally the stain on the counter.”
Now, the numbers—10-30 grams of methylene blue in 20,000 liters of water. How wide is this range? It might seem vague, but wait until you hear insider chemistry tales. In laboratory practice, once you start dealing with grams instead of milligrams, it feels like pouring a tsunami in slow motion, though in reality, it’s still just a sprinkle. Settling around 20 grams means you’re looking at 1 mg/L concentration or roughly 4.6 micromolar (μM). That tiny amount tints that massive volume with a striking blue.
Why So Potent? The Science Behind the Stain

Methylene blue sports a high extinction coefficient—around 95,000—which simply means it’s superb at absorbing and transmitting color. To put this in context, other dyes boast coefficients as high as 400,000, but 95,000 remains in the superhero league of pigments.
Plus, methylene blue’s molecular features make it stick around, especially on damaged or dead skin. Ever heard of a “blue tattoo” for a chemical spill? One lab tech did—he stuck his finger with a needle full of methylene blue while compounding an IV bag and sported a tiny blue tattoo on both sides of his finger for weeks. Not the souvenir anyone signed up for.
It stains deeper than skin, too. During open-heart surgery, methylene blue can bubble up through skin, creating a temporary internal tattoo. Visual proof that this dye does not mess around.
User Stories: When Blue Invades Your Life
Laboratory stories about methylene blue are as colorful as the dye itself. One worker once threatened to dye Lake Erie blue—for science, of course. While never acted on, that notion reflects just how powerful and impactful small quantities can be.
Another anecdote: someone dropped 10 ml of methylene blue antidote in their storage area. The floor stayed blue for six years, until a renovation erased the mark. Reminds you—chemical stains sometimes outlast the people handling them.
One chemistry enthusiast once admitted to punting an open bottle of methyl red 20 feet across the lab. The moral of the story? Dangerous compounds might double as unconventional sports equipment, but don’t try this at home.
Medical Use: From Stains to Lifesavers
Beyond playground anecdotes and pool coloring, methylene blue has serious medical credentials. It is administered intravenously at 1-2 mg/kg doses (up to 50 mg) to treat methemoglobinemia, a condition where blood struggles to carry oxygen properly.
However, it isn’t without caution. The dye is a known monoamine oxidase inhibitor (MAOI), which means it can interact with certain drugs and affect brain chemistry. A couple of curious chemistry colleagues even drank methylene blue (safely and after verifying ingestion limits) just to see if it turned their pee blue. It did. Funny, but probably not a recommended I.V. for novelty purposes.
Handling Hazards and How to Deal With Them
Handling methylene blue requires respect. It stains organic material like its name depends on it. Skin and even wounds can turn a greenish-blue hue, which might alarm the untrained eye.
Good news: stains aren’t permanent if treated properly. Applying ascorbic acid (vitamin C) or sodium ascorbate and vigorous rubbing will reduce the blue dye into a colorless form called leuco-methylene blue. It dissolves better and fades. Just remember, exposure to air will turn it blue again, so repeated washing or prevention is key.
And for your home mishaps—don’t despair if your bathtub resembles the ocean after a spill. Methylene blue’s potency means a little goes a long blue way.
Environmental Impact and Practical Considerations
Is all this blue madness safe for the environment? Methylene blue is not typically considered a pollutant despite its dramatic appearance. It colors water, yes, which counts as a water quality characteristic, but it is not toxic at low concentrations like those used in pools or water parks. Some organic compounds with similar benzene rings carry strict limits of less than 1 μg/L; methylene blue concentration at 1 mg/L far exceeds those but still isn’t formally classified as a hazard in these ranges.
But caution remains necessary. Environmental specialists wrestling with pollution find dyes challenging, as they try to remediate polluted waters. When singlet oxygen species produced by methylene blue interact with pool liners, they cause tiny holes and weaken the material. That knows no fun for pool owners expecting impermeable surfaces.
Here’s a fun thought experiment: what if you tossed 20,000 grams (yes, 20 kilograms!) of methylene blue into a 30-liter pool? It’s not just blue—it’s “smurf apocalypse” blue. Luckily, realistic scenarios keep the quantities far, far lower.
The Cultural Footprint of Blue
Methylene blue’s impact isn’t just confined to labs and waterparks. Pop culture has embraced blue in its own chemistry-tinged way. Fans of Breaking Bad instantly recognize why Walter White’s meth was blue—it’s a quirky nod to chemistry reality wrapped in storytelling.
From big fat liars to Minecraft’s virtual water, blue-tinted water captivates imaginations everywhere.
And humor? Plenty. Lab insiders joke about “bluing themselves” unintentionally, pranksters adding blue dye to communal coffee pots (everyone’s urine suddenly turning blue), or dramatic plans to create “skunk factory explosions” with chemical concoctions. While hilarious as tales, accidents remain real possibilities.
What Have We Learned?
- Methylene blue is incredibly potent. A tiny speck colors massive volumes of water.
- Handling requires care. Stains on skin and surfaces are common, and can be stubborn.
- It is medically valuable but must be dosed correctly.
- Environmental impacts exist and responsible usage is essential.
- Cultural curiosity surrounds it. Its distinct blue has inspired science, stories, and humor.
So next time you see a sparkling blue pool or hear a chemist recounts their “blue tattoo,” you’ll appreciate just how potent dyes like methylene blue truly are. Their power lies not just in color but in history, science, and sometimes, a blue hue to remind us all to be careful with chemistry.
Oh, and maybe skip drinking it for fun. Your pee might look like a Smurf’s, but your doctor might not be amused.
What does 10-30 grams of methylene blue in 20,000 liters of water mean in terms of concentration?
This equals about 1 milligram per liter at 20 grams. The solution concentration is roughly 4.6 micromolar if using 30 grams. This shows how little methylene blue is needed to color large water volumes.
Why is methylene blue considered extremely potent as a dye?
Methylene blue stains strongly even at very low amounts. Small spills, like a few milliliters, can color large areas for years. The dye has a high extinction coefficient, making it very effective at absorbing light.
Can methylene blue stain skin or other surfaces permanently?
Yes, it stains damaged or dead skin, causing blue-green marks. These stains can last weeks but may be removed with substances like ascorbic acid. On surfaces, it can persist for years if not cleaned.
Is methylene blue harmful or toxic at concentrations used in water?
At 1 mg/L or lower, it is generally not harmful and is even used medically. However, methylene blue is a known MAOI and should be handled with care. Toxicity depends on dosage and exposure.
How does methylene blue compare environmentally to other chemical pollutants?
Methylene blue itself isn’t usually considered a pollutant, but color affects water quality. Other organics with benzene rings have stricter limits, often over 1000 times lower than methylene blue levels in water.




Leave a Comment