Why Drinking Ethanol Acts as an Antidote to Swallowing Ethylene Glycol
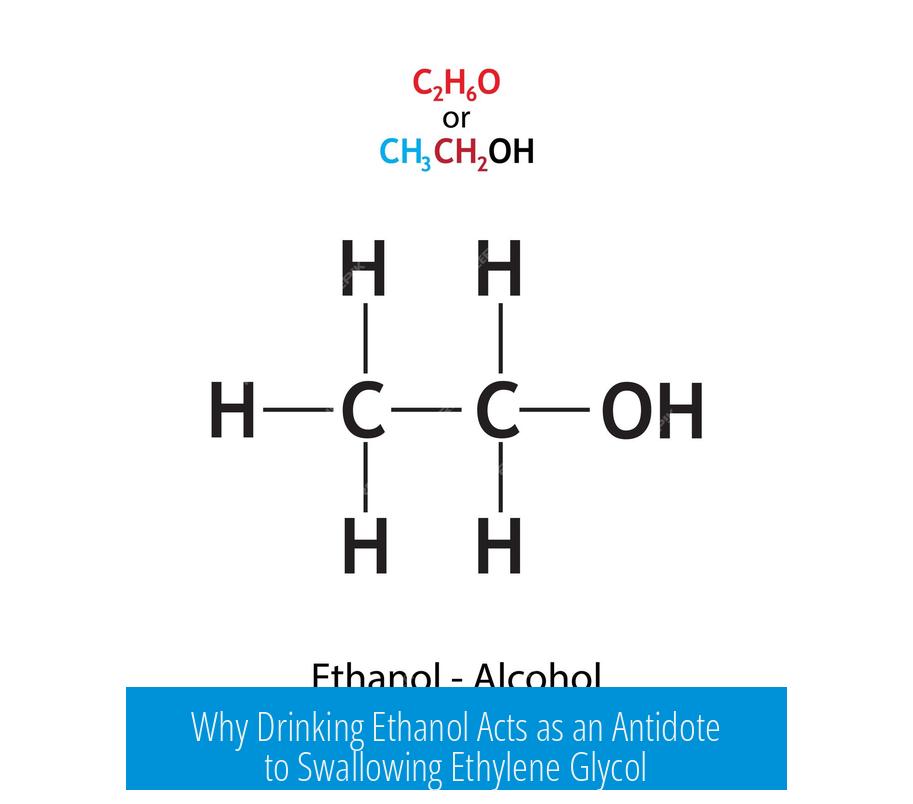
Ethanol serves as an antidote to ethylene glycol poisoning by competitively inhibiting the enzyme alcohol dehydrogenase (ADH), preventing or delaying the metabolism of ethylene glycol into toxic compounds that cause organ damage.
Understanding the Mechanism of Action
Ethylene glycol itself is relatively less toxic. The major harm arises when the body metabolizes it via the alcohol dehydrogenase enzyme. ADH converts ethylene glycol into glycolaldehyde, which is further metabolized into toxic acids such as glycolic acid and oxalic acid. These metabolites cause metabolic acidosis, kidney failure, and central nervous system damage.
Ethanol acts as a competitive inhibitor at the ADH enzyme. It competes with ethylene glycol for the enzyme’s active site because ethanol has a higher affinity for ADH than ethylene glycol does. When ethanol occupies ADH, it slows down or blocks the conversion of ethylene glycol into its harmful metabolites.
This competitive inhibition allows more time for the ethylene glycol to be excreted unchanged by the kidneys before it can cause damage. Essentially, ethanol occupies the enzyme, delaying toxic metabolite formation.
Clinical Use of Ethanol for Ethylene Glycol Poisoning
In medical settings, ethanol is employed as a legitimate antidote after ethylene glycol ingestion. The goal is to maintain high blood ethanol concentrations to saturate ADH enzymes effectively.
Providers administer ethanol intravenously or orally depending on available resources. It reduces morbidity and mortality by halting ethylene glycol metabolism. However, patients still require close monitoring and supportive care because initial absorption of ethylene glycol and partial metabolism may occur before ethanol treatment starts.
Dose Requirements and Safety Considerations
Effective inhibition of ADH demands significant ethanol levels, often requiring a substantial amount of ethanol intake. For example, achieving therapeutic ethanol plasma concentrations might demand continuous dosing over a day or more.
This poses risks: excessive ethanol can cause intoxication, respiratory depression, or alcohol poisoning. Therefore, medical oversight is crucial in dosing and monitoring ethanol therapy. Attempting to self-treat with ethanol after antifreeze ingestion without medical guidance is dangerous and strongly discouraged.
Comparing Toxicity: Ethylene Glycol vs. Ethanol
Both ethylene glycol and ethanol exhibit toxicity, but their harm differs in mechanism and severity. Ethylene glycol toxicity primarily arises from its metabolites, which are more damaging than the parent compound. Ethanol itself is somewhat toxic but usually less harmful at moderate doses and is metabolized differently.
Blocking ethylene glycol metabolism with ethanol prevents formation of key toxic metabolites, significantly reducing the risk of severe toxicity and organ failure.
Similarity to Methanol Poisoning Treatment
The antidotal use of ethanol for ethylene glycol poisoning parallels its use in treating methanol poisoning. Both compounds are metabolized by alcohol dehydrogenase into toxic metabolites (formaldehyde from methanol; glycolic and oxalic acids from ethylene glycol).
Ethanol competitively inhibits ADH, preventing or delaying the formation of these metabolites in both poisonings. Thus, ethanol remains a cornerstone for managing these toxic alcohol ingestions.
Summary Table: Key Facts on Ethanol as Antidote for Ethylene Glycol Poisoning
| Topic | Description |
|---|---|
| Mechanism of Action | Ethanol competitively inhibits alcohol dehydrogenase, blocking ethylene glycol metabolism. |
| Clinical Use | Used in hospitals to maintain high ethanol levels and prevent toxic metabolite formation. |
| Dosing and Safety | Requires substantial ethanol intake; risk of alcohol poisoning necessitates medical supervision. |
| Toxicity Comparison | Ethylene glycol metabolites cause severe toxicity, whereas ethanol itself is less harmful at moderate levels. |
| Similarity to Methanol Treatment | Mechanism and treatment approach are similar due to shared enzyme metabolism. |
Key Takeaways
- Ethanol competitively inhibits ADH, preventing ethylene glycol metabolism into toxic acids.
- This inhibition reduces or delays organ damage caused by ethylene glycol metabolites.
- Clinical treatment involves maintaining therapeutic ethanol blood levels under medical supervision.
- Large ethanol doses increase risks of intoxication; self-treatment is unsafe and not advised.
- The treatment approach mirrors that used in methanol poisoning due to similar metabolic pathways.
Can Someone Explain Why Drinking Ethanol Acts as an Antidote to Swallowing Ethylene Glycol (Antifreeze)?
Simply put: Drinking ethanol works as an antidote to ethylene glycol poisoning because it blocks the enzyme that turns ethylene glycol into deadly toxins. This sounds like a chemistry class cocktail party, but it’s a fascinating and lifesaving interaction. Let’s dive in!
Meet the Villain: Ethylene Glycol and Its Toxic Metabolites
Ethylene glycol itself isn’t the main troublemaker when swallowed; it’s the nasty byproducts it turns into inside your body. When ethylene glycol enters your system, an enzyme called alcohol dehydrogenase (ADH) gets to work breaking it down. Unfortunately, this process produces toxic substances like glycolic acid and oxalic acid, which damage your kidneys, brain, and other organs.
So, if ethylene glycol itself isn’t overly toxic, why the panic? Because once those metabolic byproducts form, the real damage begins. Ingesting antifreeze is a race against time to stop this enzyme-driven destruction.
The Hero: Ethanol as a Competitive Inhibitor
Here’s where ethanol—the drinking alcohol in vodka, beer, and wine—steals the show. Ethanol also uses ADH as its metabolic gateway. When ethanol is present, it competes with ethylene glycol for the enzyme’s attention. Imagine ADH as a bouncer at a club letting customers inside one at a time. If ethanol gets in first, ethylene glycol has to wait.
This “waiting game” is not just a slow dance; it’s a lifesaving delay. The enzyme prioritizes ethanol metabolism, which slows the generation of the toxic ethylene glycol byproducts. This buys precious time to clear the ethylene glycol from the system through urine before it wreaks havoc.
“I learned about this in organic chemistry 2. It’s the byproducts caused by the alcohol dehydrogenase enzyme breaking down ethylene glycol that damages your organs. Drinking ethanol means the ethanol and ethylene glycol have to compete for available enzyme.”
Clinical Use: Ethanol in the Emergency Room
Medical professionals take advantage of this competitive inhibition. In cases of confirmed ethylene glycol poisoning, hospitals administer ethanol intravenously or orally. This clinical protocol helps ensure enough ethanol is circulating in the bloodstream to outcompete ethylene glycol effectively.
But don’t get any ideas about self-medicating by drinking a few shots at home! The therapeutic dose of ethanol is quite high, and managing it without medical supervision can cause serious alcohol poisoning.
Consider this excerpt from a hospital story: a patient tried to commit suicide by swallowing antifreeze but drank vodka too. They survived, likely due to the ethanol competing with the ethylene glycol, but their blood’s serum osmolality—the concentration of substances dissolved in blood—was dangerously high. The patient faced long-term complications despite survival.
“Had a person come into the hospital ER I used to work for. They drank antifreeze with vodka. They did not die, but their serum Osmolality was the highest I’ve ever seen. I’d be surprised if they survived without very harsh long term effects.”
You Need the Right Dose: Ethanol Is No Light Snack
The crucial point here: to be an effective antidote, ethanol levels in the blood must be high enough to saturate the ADH enzymes. This means ingesting quite a substantial amount of ethanol, which carries its own risks.
“You’d have to drink a decent amount of ethanol to cancel out the ethylene glycol… don’t overdose on the ethanol or you may get alcohol poisoning.”
So, even if ethanol is your lifeguard in the toxic sea of antifreeze, it’s a lifeguard with strict rules. Doctors carefully calculate and monitor ethanol dosing to balance efficacy and safety. DIY ethanol therapy is a dangerous game that could result in alcohol poisoning, coma, or death.
Comparison: Ethylene Glycol Toxicity vs. Ethanol Toxicity
Interestingly, ethylene glycol itself has about the same inherent toxicity as ethanol. The real issue is the metabolic aftermath. Ethylene glycol’s break down products lead to acidosis and kidney failure, while ethanol metabolism is relatively safer under controlled conditions.
Therefore, the body has no particular problem handling ethanol—it just temporarily prioritizes breaking down ethanol over ethylene glycol to prevent poison formation. This little metabolic prioritization trick explains ethanol’s role as an antidote.
“Ethylene Glycol itself has the same toxicity as Ethanol basically, but breaks down into more toxic metabolites, unless the metabolism has been inhibited by Ethanol.”
Bonus Tidbit: Ethanol Saves the Day for Methanol Poisoning, Too!
The wisdom of ethanol as an antidote doesn’t stop with ethylene glycol. The exact same mechanism applies to methanol poisoning. Methanol, when metabolized, produces formaldehyde and formic acid, which can cause blindness and death. Ethanol competes for ADH, delays methanol metabolism, and thus helps prevent these horrible effects.
“Same rule applies for methanol poisoning as well. It’s also the antidote to methanol poisoning.”
Practical Advice and Warnings (But Please Don’t Try This at Home)
There exists Internet folklore on “how to survive antifreeze ingestion” by mixing antifreeze with ethanol-containing beverages. Here’s the gist, though it’s highly discouraged and unsafe: some suggest diluting the antifreeze, drinking it quickly, waiting for intoxication, then continuously drinking ethanol to block metabolism of ethylene glycol.
Sounds like a dangerous party trick. The risks include severe poisoning from both chemicals, alcohol overdose, and unpredictable outcomes. Medical supervision is critical.
“If you want to safely drink antifreeze, mix the antifreeze with some water or soda, then quickly drink it, wait until tipsy, then drink shots of ethanol per hour. But don’t overdose on the ethanol or you may get alcohol poisoning. Try to stay consistent with ethanol for at least 24 hours.”
Warning: This is NOT a safe or recommended practice under any circumstances. Seek medical help immediately if exposure occurs.
Final Thoughts: The Chemistry Behind Safety
The story of ethanol as an antidote vividly illustrates how detailed biochemical knowledge saves lives. By understanding the enzymes and metabolic pathways, we can cleverly block poison formation and buy time for treatment.
So, the next time someone asks why drinking ethanol helps after swallowing antifreeze, you can confidently say: it’s a fierce enzymatic competition, where ethanol rushes in to shield your organs by keeping ethylene glycol at bay.
Just remember: this is a medical treatment, not a recommendation to gulp down vodka after a mishap. Responsible use under medical observation is the key to survival.
Why does ethanol work as an antidote to ethylene glycol poisoning?
Ethanol competes with ethylene glycol for the enzyme alcohol dehydrogenase (ADH). This delays or blocks ethylene glycol’s breakdown into toxic compounds that cause organ damage.
How is ethanol used in hospitals for ethylene glycol poisoning?
Hospitals give ethanol to maintain high blood levels, which stops ethylene glycol metabolism. This treatment reduces toxicity and can prevent death but may not fully avoid long-term effects.
Is drinking ethanol at home a safe way to treat ethylene glycol ingestion?
No, effective treatment requires careful dosing to avoid ethanol poisoning. Self-treatment with alcohol is risky and not medically recommended.
How toxic is ethylene glycol compared to ethanol?
Ethylene glycol and ethanol have similar toxicity initially. The danger comes from ethylene glycol’s metabolites formed during metabolism, which ethanol blocks from forming.
Does ethanol work the same way for methanol poisoning?
Yes, ethanol inhibits the same enzyme (ADH) for methanol. It prevents toxic methanol metabolites from forming, using the same competitive inhibition mechanism.




Leave a Comment