Why Is Bond Dissociation Energy Higher for C=O in CO2 Than in Other C=O Bonds?
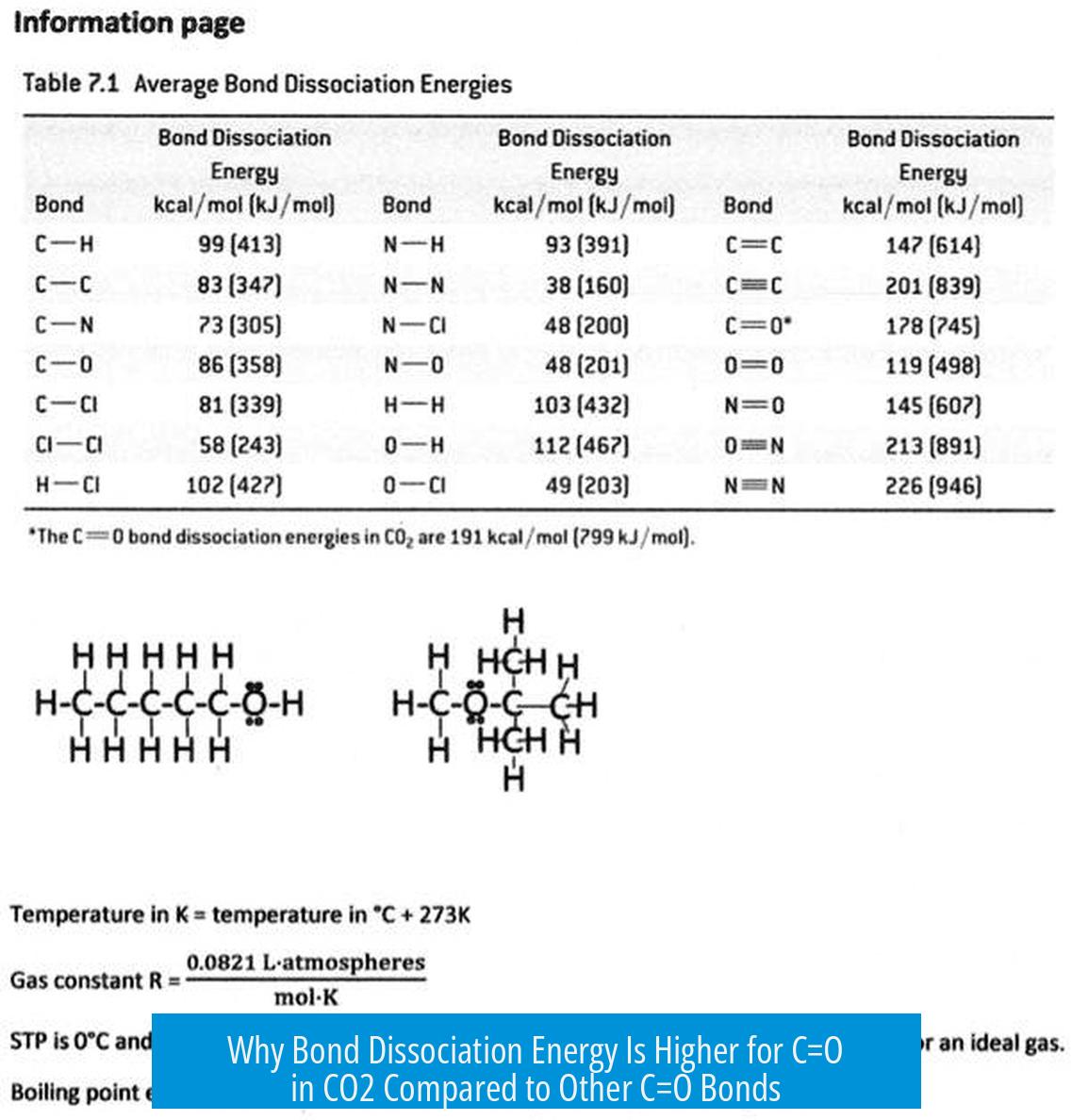
The bond dissociation energy (BDE) of the C=O bond in carbon dioxide (CO2) is higher than in most other carbonyl compounds due to its unique electronic and structural properties that increase bond stability. This includes nonpolar bonding, electron delocalization over the molecule, and an even electronegativity distribution, all of which make the double bonds in CO2 stronger and harder to break.
Nonpolar Nature of C=O Bonds in CO2
In CO2, the two C=O bonds are effectively nonpolar. This contrasts with typical carbonyl groups (e.g., in aldehydes and ketones), where the C=O bond is polar due to differences in electronegativity between carbon and oxygen.
- The nonpolar character results from the linear, symmetric structure of CO2.
- Both oxygens withdraw electron density equally, preventing localized electron deficiency at carbon.
- This balanced electron distribution enhances bond stability.
Electron Delocalization and Molecular Geometry
CO2 exhibits significant electron delocalization. The valence electrons are shared across the carbon and two oxygens rather than confined to one C=O bond.
- Electron density is spread over the molecule, lowering energy and increasing bond strength.
- The linear geometry with a 180° bond angle supports optimal p-orbital overlap for π-bonding.
- This delocalization gives CO2 bonding characteristics somewhat akin to aromatic systems, enhancing stability beyond typical carbonyl bonds.
Electronegativity Distribution and Polar Character
Oxygen’s high electronegativity induces a polar character in most carbonyl bonds, making the carbon electrophilic and the bond less stable.
In CO2, the symmetrical arrangement spreads charge evenly. The even electronegativity distribution reduces localized charge buildup, which stabilizes the molecule and increases the energy required to break the bonds.
Comparison to Typical Carbonyl Bonds
| Property | C=O in CO2 | C=O in Aldehydes/Ketones |
|---|---|---|
| Bond Polarity | Nonpolar (symmetric) | Polar (asymmetric) |
| Electron Delocalization | High (over both oxygens) | Localized on carbonyl carbon |
| Geometry | Linear (180° angle) | Bent or trigonal planar (often <120°) |
| Bond Stability | High | Lower |
These differences culminate in a higher bond dissociation energy for CO2’s C=O bond than in other carbonyl-containing molecules.
Key Takeaways
- CO2 C=O bonds are nonpolar due to symmetric electronegativity distribution, enhancing stability.
- Electron delocalization across carbon and both oxygens increases bond strength in CO2.
- The linear geometry optimizes π-overlap, resembling features of aromatic systems.
- Typical carbonyl bonds are polar and localized, making them easier to break.
Why is the C=O bond in CO2 more stable than in other carbonyl compounds?
The C=O bond in CO2 is non-polar, making it more stable. In contrast, most other carbonyl bonds (RC=O) are polar and less stable, so CO2’s bond needs more energy to break.
How does electron delocalization increase the bond dissociation energy in CO2?
Electrons are spread between carbon and the two oxygens in CO2. This delocalization strengthens the bonds, similar to aromatic systems, raising the energy needed to break the C=O bonds.
What role does the bond angle in CO2 play in its bond strength?
CO2’s 180-degree bond angle allows optimal overlap of orbitals. This linear shape aids electron delocalization and stabilizes the C=O bonds, increasing bond dissociation energy.
How does electronegativity affect the stability of the C=O bond in CO2?
Carbon and oxygen have more balanced electronegativities in CO2. This even electron distribution reduces polarity and makes the bond stronger compared to more polar carbonyl groups.
Why do polar C=O bonds in other compounds usually have lower bond dissociation energy?
In polar carbonyl bonds, oxygen pulls electrons from carbon more strongly. This electron deficiency makes the bond less stable and easier to break, lowering bond dissociation energy compared to CO2.




Leave a Comment